- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Critical Review on Thalassemia: Types, Symptoms and Treatment
Hamidreza Shirzadfar*, Nasim Mokhtari
Department of Biomedical Engineering, Sheikhbahaee University, Esfahan, Iran
*Corresponding author: Dr. Hamidreza Shirzadfar, Department of Biomedical Engineering, Sheikhbahaee University, Esfahan, Iran
Submission: March 16, 2018; Published: April 20, 2018

ISSN 2640-9275 Volume1 Issue2
Abstract
Thalassemia is a genetic blood disorder where the normal hemoglobin protein is produced in lower amounts than usual and share in common one feature. This means it is passed down from one or both parents through their genes. People with Thalassemia are not able to make enough normal hemoglobin, which causes severe anemia. Hemoglobin is found in red blood cells and carries oxygen to all parts of organ in the body; therefore organs are unable to function properly. There are 30 million carriers and approximately 10000 children are born with the disease every year in the world. There are two main classes of thalassemia, Different genes are affected for each type in your body. α and β, in which the α-globin and β-globin genes are involved. In this study we will generally explain thalassemia disease, types of it and its treatment.
Keywords: Thalassemia; Beta globin; Anemia; Blood transfusions; Deferoxamine; Globin gene; Deferiprone
Introduction
Thalassemias or Mediterranean anemia comes from the Greek words “Thalassa” meaning sea, and “Emia” meaning blood, was described in 1925 by a physician who studied Italian children with severe anemia, early childhood death and huge abdominal organs, and caused by impaired synthesis of one or more globin chains of the hemoglobin (consisting of 4 polypeptide chains), resulting in much less oxygen being bound to the hemoglobin molecules and transported through the body [1,2]. Depending on which polypeptide chains are affected, the thalassemias are named α, β, γ or δ thalassemia. It is the β chain that is most frequently affected, so that this disorder is called β-thalassemia. β-thalassemia major is a homozygous form in which both alleles are severely mutated so that β chain synthesis is stopped completely, whereas β-thalassemia minor is a heterozygous form resulting in an approximately 20% reduction of polypeptide synthesis. To compensate this reduction, more HbA2 and HbF are produced: in β-thalassemia major it is more HbF, and in β-thalassemia minor primarily HbA2. In 1946, the cause of thalassemia was found to be an abnormal hemoglobin structure. The body reacts by destroying red blood cells, causing anemia. To compensate for this deficiency, the body tries to make more red blood cells faster, resulting in other thalassemia complications such as bone disorders, spleen enlargement, and heart problems. In the 1960s, doctors discovered a new way to treat thalassemia, and began replacing fresh blood instead of patient blood every month. This method was most commonly used for patients with thalassemia major and is still used to treat the diseases. But, after each passing blood transfusion, the body encountered an increased amount of iron that could not be removed naturally. As a result, most patients with thalassemia died for the same reason. The researchers later discovered the drug to remove excess iron from the body by treatment with a drug called deferoxamine [3].
This drug prevented iron-inducted heart disease and helped patients live much longer. Recently, two oral drugs have dramatically improved the quality of life of patients with iron overload from transfusions for thalassemia. Furthermore, In Iran as a premarital screening, the man’s red cell indices are checked first, if cell hemoglobin < 27pg or red cell volume < 80fl, the woman is tested at last. When both have this feature, their hemoglobin A2 concentrations are measured. If both have a concentration above 3.5% of hemoglobin A2, (diagnostic of thalassemia trait) they are referred to the local designated health post for genetic counseling [4]. People with thalassemia minor, if malaria is diagnosed, their likelihood of death is lower than that of others. Therefore, thalassemia minor has a great advantage. Of course, malaria treatment does not eliminate thalassemia. In this research, the natural structure of the hemoglobin gene and the various types of thalassemic disease will be described, then the signs and symptoms of the disease, and finally the treatment of the disease will be explained.
Normal structure and expression of globin gene clusters
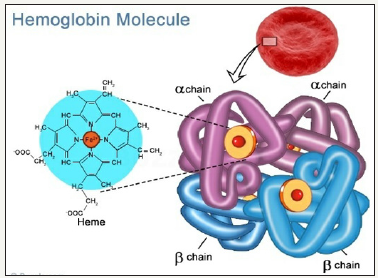
Human hemoglobin is a heterotetramer protein, compose of two alpha and two beta subunits as shown in Figure 1. Each subunit contains a heme group, an iron containing compound that binds to oxygen [5]. The synthesis of hemoglobin is controlled by two developmentally regulated multigene clusters: the alpha-like globin cluster on chromosome 16 and the beta-like 9.5 chromosome 11. In healthy persons, the synthesis of alpha and beta globin chains is finely balanced during terminal erythroid differentiation but the mechanism of balanced expression is unknown [6,7].
Figure 1: Hemoglobin structure.
Alpha thalassemia
Each human diploid cell contains four copies of the alphaglobin gene, located on chromo-some [8]. Alpha thalassemia is the result of reduction in the synthesis of the alpha globin chains and a form of thalassemia involving the gene HbA1 and HbA2 [9,10]. Two main types of alpha thalassemia are described as alpha thalassemia major and hemoglobin H disease that Alpha thalassemia major is a very serious disease of severe anemia that begins even before birth. Most affected babies do not survive full gestation or die shortly after birth. Hemoglobin H disease is milder than beta thalas
Beta thalassemia
The most familiar type of thalassemia is beta thalassemia. Thalassemia was first recognized clinically in 1925 when Thomas Cooley described a syndrome of anemia, splenomegaly, and bony deformities among Italian descents [11]. Beta thalassemia or Cooley’s anemia is caused by a change in the gene for the beta globin component of hemoglobin. Beta thalassemia is caused by damaged or missing genes. Thalassemia can be subjected to modification in the hemoglobin genes depending on the mild and mild side effects [12]. Beta thalassemia patients is most found in people who are from Greek, Italian, African, or Asian origin especially India. Beta thalassemia major has begun since childhood and will last until the end of the life. The severe anemia can result in severe lethargy, paleness, and insufficient growth and development. Other characteristic physical complications such as heart problems and excessive liver and spleen growth which decrease life-expectancy. This condition is becoming more prevalent in the USA as a result of Asian immigration.
Signs and symptoms of thalassemia
Iron overload: The most common complications related to patients on regular transfusion are iron overload. People with thalassemia can get an overload of iron in their bodies, and too much iron can result in damage to the heart, liver, and endocrine system.
Infection: People with thalassemia have an increased risk of infection and this happen is so dangerous for organs of the body.
Bone deformities: In this disease, the natural development of the body is affected. Consequently, it may be observed in patients with thalassemia. In most cases, skull bone is seen. The bones of the face and the skull become thicker, and in addition to skeletal malformations.
Enlarged spleen: Enlargement of the spleen has more infectious, viral and bacterial causes, and is secondarily due to bugs in the blood flow and liver failures. That is, if the liver becomes inflamed, it will squeeze the spleen. Thalassemia is one of the diseases that lead to enlargement of the spleen.
Symptoms like anemia: For example, Shortness of breath, Cold hands and feet, pale skin, Irritability, Dark urine and Fatigue.
Management of thalassemia
Patients with thalassemia gradually accumulate high levels of iron (Fe) in their bodies. This build-up of iron may be due to the disease itself. This overload of iron brings with it many biochemical complications. Two key substances involved in iron transport and storage in the body are ferritin and transferrin. Ferritin is a protein present within cells that binds to Fe(II) and stores it as Fe(III), and Transferrin is an iron-binding protein present in blood plasma and carrying iron through blood [13,14]. Treatment of thalassemia depends upon the level of severity. For mild forms of the condition, advice and counseling are usually all that are necessary. For more severe forms, treatment may consist in blood transfusion; using drugs such as deferoxamine, deferiprone, or deferasirox to prevent the breakdown of hemoglobin, Deferoxamine, deferiprone and deferasirox are the three most widely used iron-chelating agents, or a bone marrow transplant using material from a compatible donor, or from the patient’s mother.
Deferoxamine (DFO)
Deferoxamine was the first drug made available for thalassemia treatment [15]. The drug deferoxamine, also known as desferoxamine B and DFO-B. It binds iron, decreasing the toxic reactions catalysed, and it also decreases the uptake of iron by tissues [13]. Pediatric patients treated with DFO should be monitored for growth delay by assessing body weight and growth every 3 months and Patients treated with DFO should be followed up with yearly assessments of vision and auditory function [16]. Deferoxamine is administered via intravenous, intramuscular, or subcutaneous injections [17]. Deferoxamine was also shown to improve liver function by arresting the development of hepatic fibrosis [18] and another endocrine abnormality that thalassemic patients face is diabetes mellitus, which results from iron overload in the pancreas impairing insulin secretion.
Deferiprone (DFP)
DFP, the first oral iron chelator to be used, is approved in Europe and other countries for transfusional iron overload in patients, when DFO therapy is inadequate [19]. DFP is synthetically made and is highly selective to Fe (III) [20,21]. Deferiprone is an iron chelator that is orally active, its administration thus being easier than that for deferoxamine [20-22]. When comparing deferiprone to deferoxamine, it should be noted that they both bind iron with similar efficiency. DFP was also found to be significantly more effective than deferoxamine in treating myocardial siderosis in patients with thalassemia [20]. DFP may also cause arthropathy, increased liver-enzyme levels, progression of hepatic fibrosis associated with increase in iron overload or hepatitis C, and low plasma zinc level [19,23]. DFP is also smaller than deferoxamine. In a study after combined treatment with deferoxamine and deferiprone in thalassemia major patients, echocardiography showed a significant improvement in systolic and diastolic function of the heart. Therefore, the use of the above combination therapy has clearly improved cardiac performance and increased survival rates in patients.
Deferasirox (DFX)
Deferasirox has good oral bioavailability and a long half-life suitable for once-daily dosing. Deferasirox is capable of removing iron from the blood through the coordination of two molecules of the deferasirox to a single iron ion [24]. The ability of deferasirox to remove iron stems directly from its relatively small size, which is what allows it to access the iron contained within the blood and inside tissues. An additional benefit of the use of deferasirox instead of desferoxamine is that, unlike desferoxamine, early studies have indicated that deferasirox does not have a significant impact on the growth and development of pediatric thalassemia patients [25]. The safety profile of deferasirox is similar in pediatric and adult patients. A once-daily dose of 20mg/kg of body weight has been found to be sufficient for patients for the maintenance of liver iron concentration (LIC) levels, which are measured as mg of iron per g of liver tissue. As with deferoxamine, deferasirox doesn’t work if the patient does not take it [26].
Bone marrow transplant
Bone marrow transplantation may offer the possibility of a cure in young people who have an HLA-matched donor [27]. A stem cell transplant may be an option in select cases, including children born with severe thalassemia. In low-risk young patients, the thalassemia-free survival rate is 87%; the mortality risk is 3% [28]. If the patient does not have an matched compatible donor, there is another curative method called Bone Marrow Transplantation from haploidentical mother to child [29]. Prior to a stem cell transplant, you receive high doses of drugs or radiation to destroy your diseased bone marrow. Then you receive infusions of stem cells from a compatible donor. However, because these procedures have serious risks, including death, they’re generally reserved for people with the most severe disease who have a well-matched donor available.
Future Direction
Medications that increase fetal hemoglobin in thalassemia have greatly improve life for patient suffering from this disease; however safer and more effective drugs are still being sought. Stem cell transplantation can be used to treat the illness, but it has many limitations. Also an ideal iron chelator would have high iron chelating efficiency, high oral availability, tolerable profile of adverse events, once-daily dosing, palatable formulation, and high penetration into organs with iron deposition. Further research to improve the safety of transplantation, especially when using stem cells from unrelated donors.
References
- Shirzadfar H, Mokhtari N, Claudel J (2018) geometric parameters optimization of interdigital micro-electrodes: theoretical analysis. Accepted by Journal of Nano and Electronic Physics
- Uthman MD (2007) Hemoglobinopathies and thalassemias.
- Brittenham, GM, Patricia GM, Arthur NW, Christine ME, Young NS, et al. (1994) Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. New England Journal of Medicine 331(9): 567–573.
- Samavat A, Modell B (2004) Iranian national thalassaemia screening programme. BMJ 329(7475): 1134–1137.
- Maton A, Hopkins, McLaughlin GW, Johnson S, Warner MQ, et al. (1993) Human biology and health. Englewood Cliffs, New Jersey, USA.
- Steensma DP, Gibbons RJ, Higgs DR (2005) Acquired α-thalassemia in association with myelodysplastic syndrome and other hematologic malignancies. Blood 105(2): 443-452.
- Lehmann H, Carrell RW (1968) Differences between alpha and beta chain mutants of human haemoglobin and between alpha and beta thalassaemia, Possible duplication of the alpha chain gene. Br Med J 4(5633): 748–750.
- Hemoglobin-alpha locus 1 HBA1. Online mendelian inheritance in man (OMIM) 141800.
- Hemoglobin-alpha locus 1 HBA2. Online mendelian inheritance in man (OMIM) 141850.
- Lukens JN (1993) The thalassemias and related disorders: quantitative disorders of hemoglobin synthesis. Bayl Univ Med Cent 20(1): 27-31.
- Nigam N, Nigam S, Agarwal M, Singh PK (2015) β-Thalassemia: From clinical symptoms to the management IJCMR 4(5): 1-5.
- Brittenham GM, Olivieri NF (1997) Iron-chelating therapy and the treatment of thalassemia. Journal of the American Society of Hematology 89(3): 739–761.
- Aisen P, Leibman A, Zweier J, Zweier L (1978) Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem 253(6): 1930–1937.
- Thalassemia International Federation: Guidelines for the clinical management of thalassemia (2nd edn).
- Novartis (2011) Desferal (Deferoxamine Mesylate) Prescribing Information. Novartis Pharmaceuticals, Switzerland, pp. 1-8.
- Alan C, Marie M, Elias S (1981) Response to long-term deferoxamine therapy in thalassemia. The Journal of Pediatrics 99(5): 689–694.
- Haiyan J, Shuji T, Isao S (2007) The iron chelator deferoxamine causes activated hepatic stellate cells to become quiescent and to undergo apoptosis. Journal of Gastroenterology 42(6): 475–484.
- Agency EM (2009) Ferriprox: EPAR – Product Information.
- Viroj W (2006) Quantum chemical analysis of the deferiprone-iron binding reaction. International Journal of Nanomedicine 1(1): 111–113.
- Olivieri, NF, Brittenham GM (1997) Iron-Chelating Therapy and the Treatment of Thalassemia. Blood 89(3): 739–761.
- Galanello R, Campus S (2009) Deferiprone Chelation Therapy for Thalassemia Major. Acta Haematologica 122(2–3): 155–164.
- Olivieri NF, Brittenham GM, McLaren CE (1998) Long-term safety and effectiveness of iron-chelation therapy with deferiprone for thalassemia major. N Engl J Med 339(7): 417–423.
- Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, et al. (2006) A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood 107(9): 3455–3462.
- R Galanello, Piga A, Forni GL, Bertrand Y, Foschini ML, et al. (2006) Phase II clinical evaluation of deferasirox, a once-daily oral chelating agent, in pediatric patients with β-thalassemia major. Haematologica 91(10): 1343–1351.
- Aydinok Y, Kattamis A, Cappellini MD (2013) Deferasirox– deferoxamine combination therapy reduces cardiac iron with rapid liver iron Removal in patients with severe transfusional iron overload (Hyperion). 55th ASH annual meeting, Los Angeles, USA 122: 2257.
- Argiolu F, Sanna MA, Addari MC (1997) Bone marrow transplantation in thalassemiam, The significance of Cagliari. Bone Transplant 19(2): 1-65.
- Sabloff M, Chandy M, Wang Z, Logan BR, Ghavamzadeh A, et al. (2011) HLA-matched sibling bone marrow transplantation for β-thalassemia major. Blood 117(5): 1745–1750.
- Sodani P, Isgrò A, Gaziev J, Paciaroni K, Marziali M, et al. (2011) T celldepleted hla-haploidentical stem cell transplantation in thalassemia young patients. Pediatric reports 3(2): e13.
- Cappellini MD, Cohen A, Porter J, Taher A, Viprakasit V (2014) Guidelines for the Management of Transfusion Dependent Thalassemia. (3rd edn), Cyprus Thalassaemia International Federation, Cyprus.
© 2018 Dr. Hamidreza Shirzadfar. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






