- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Sensible Use of Technologies to Increase Solubility and Bioavailability in Formulation Development
Mirza R Baig1*, Aliasgar Shahiwala2 and SA Khan3
1Department of Clinical Pharmacy & Pharmacotherapeutics, Dubai Pharmacy College, UAE
2Department of Pharmaceutics, Dubai Pharmacy College, UAE
3Department of Pharmaceutical Chemistry, Dubai Pharmacy College, UAE
*Corresponding author: Mirza R Baig, Department of Clinical Pharmacy & Pharmacotherapeutics, Dubai Pharmacy College, Dubai, UAE
Submission: February 13, 2018 Published: March 26, 2018

ISSN 2640-9275 Volume1 Issue1
Introduction
In drug development process, the formulation of drug and its bioavailability are the major concerns in getting approval to conduct clinical trials. A poor bioavailability and solubility property of a product leads to rejection and fail to reach the market. Poor bioavailability of drugs is due to various factors for example aqueous solubility, drug permeability, dissolution rate, first-pass metabolism, etc. [1]. The most leading factor of poor bioavailability is the low solubility and permeability. Therefore, there are extensive researches to study the Biopharmaceutics Classification System (BCS) class II, III and IV and to develop new technologies to overcome the problem of poor solubility and/or permeability. Alteration in drug delivery process may produce substantial changes in bioavailability and solubility of a drug. There are array of cutting edge techniques which are developed and have been used by some pharmaceutical companies to enhance the solubility and improve bioavailability. Identifying the need of appropriate technologies at the earlier stages of drug discovery may increase the chances of overall success rate of approval which in fact saves unnecessary expenditure and reduce the cost of drug development. The degree of solubility of a drug in a definite solvent is measured as the saturation concentration where adding more solute does not increase its concentration in the solution [2]. Different pharmacopoeias have defined solubility regardless of solvent used, in terms of quantification and defined the criteria as given in Table 1 [3].
Table 1: USP solubility criteria.

Techniques for Improving Bioavailability
Oral Bioavailability is the common route of administration and is always a challenging approach in improving bioavailability. Apart from oral bioavailability, other approach to improve the systemic availability of the drug is to deliver it by using different routes of administration based on the formulation such as injections, suppositories, pessaries, transdermal patches etc. [1]. Some of the major techniques to improve bioavailability are presented in the Table 2.
Table 2: Cutting edge technologies to improve bioavailability.
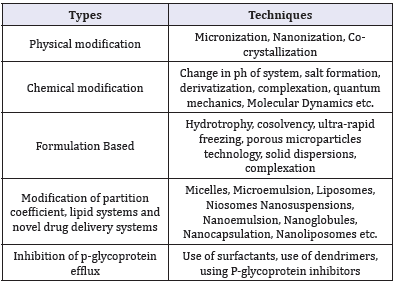
Physical modifications
Micronization: Decreasing particle size of drugs causes increase in surface area which improves the rate of dissolution. Micronization reduces the particle down to micrometer or nanometer which increases the bioavailability of poorly soluble active ingredient. A wide range of drug types which includes injectable, medicated drops, inhaled products, solid dosage are benefited from micronization. Drugs having high dose number is not suitable for micronization as it does not change the saturation solubility of the drug [1]. Micronization is usually achieved by spray-drying, freeze-drying, crystallization or milling processes. Ultrasonic energy in the range of 20kHz-5MHz is also used to increase nucleation rate, which not only induces the crystallization, but also provide reduced particle size with narrow size distribution. As it uses ultrasonic energy, this process is called sono crystallization [2].
Nano sizing: It is the reduction of particle size to the submicron range (100 to 200nm), which provide further enhancement in the dissolution rates. Supercritical fluid (SCF) technology is increasingly used for this purpose. In this technique, Drug particles are first dissolved in the SCF like carbon dioxide, nitrous oxide, ethylene, propylene, propane, n-pentane, ethanol, ammonia, and water, followed by bring rapid expansion of supercritical solutions either by moderate changes in temperature and/or pressure. Nano-suspensions with particle diameter between 5-2,000nm diameters are generated using SCF. The nano-suspension provides higher dissolution rates of particles compared to conventional suspensions. Several enhancements in SCF technology such as supercritical anti-solvents processes (SAS), impregnation or infusion of drug with polymers, solution enhanced dispersion by SCF (SEDS), and aerosol supercritical extraction system (ASES) have been recently proposed [3,4].
Chemical modification
Derivatization: Derivatization is a technique where the active ingredient is transformed into a product of similar chemical structure called derivative. A pro drug is formed for improving the aqueous solubility which covalently bound to inactive moiety and provides desired pharmacological effect [5].
Quantum mechanics and molecular dynamics: Quantum mechanics and molecular dynamics are powerful simulation tools which provide insight into molecular-level behavior and interactions between drugs and excipients. Direct calculation and visualization of the interactions provide valuation information in formulation development. With the understanding of drugexcipient interactions it is possible to select and design better molecules [6].
Formulation based techniques
Solid dispersions [sds]: Solid dispersion refers to the system comprising one or more active ingredients dispersed in an inert carrier in a solid state. SD is basically a dispersion of one or more active ingredients with the inert carrier/s and based on the carrier/s used, SD is classified into first generation (crystalline carriers such as urea and sugar), second generation (amorphous carriers usually polymers) and third generation (carriers with surface activity or self-emulsifying properties, usually contains surfactant or a mixture of amorphous polymer and surfactant). They are commonly prepared by the melting (fusion) method, solvent method, or fusion solvent-method [6]. Recently, new methods including melt extrusion [7], spray drying [8], freeze drying [9] and supercritical fluid technology [10]. Third generation SD of praziquantel using polyethylene glycol (PEG) 4000 as a hydrophilic polymer and poloxamer 188 as a surfactant showed a promising results in improving the oral bioavailability of the drug [11]. In another study, third generation amorphous solid dispersions (solid solutions) of indomethacin with poly (vinylpyrrolidonevinyl acetate copolymer) and Poloxamer 407 showed increased solubility of the API [12].
Eutectic mixtures are also a form of solid dispersions prepared by fusion method using specific solvents as the basis of a formulation strategy to improve the physical properties of the active ingredient for an enhanced dissolution rate and solubility. But not every drug and compound combination are capable of forming a eutectic mixture. The inability to predict the eutectic system and the time required to characterize these mixtures made this technology challenging to use in drug development [13]. Surface Solid Dispersion (SSD) is as the name suggest, drug is deposited on the surface of an inert water insoluble hydrophilic carrier with high surface area such as microcrystalline cellulose, hydrophilic fumed silica (CAB-O-SIL® and CAB-O-SPERSE®), crospovidone, polyvinyl pyrrolidone-vinyl acetate copolymer, sodium starch glycolate, resulting into particle size reduction of the drug. In contact with water, the carrier immediately disperses which allows rapid release of the drug and improves its rate of dissolution [14]. In a recent comparative study between SSD of meloxicam was prepared with crospovidone showed higher dissolution rates compared to SD of meloxicam prepared with PEG4000 [15].
Cyclodextrins: These starch derivatives are used widely to enhance the solubility by their ability to form non-covalent dynamic inclusion complexes in solution. Hydroxypropyl-β-cyclodextrin (HP-β-CD) is most commonly used in pharmaceutical formulation due to its high water solubility [16]. Selected examples of different cyclodextrin derivatives used are provided in Table 3 [16-29].
Modification of partition coefficient, lipid systems and novel drug delivery systems
Lipid systems: Lipid formulations are diverse group of formulations including lipid solutions, emulsions, micro-emulsion, nano-emulsion with self-Emulsifying, self-micro emulsifying, and self-nano-emulsifying drug delivery systems are recent addition to it. Lipid formulations are broadly classified into 4 categories with increasing hydrophilicity: Type I includes only oil, Type II includes oil and water insoluble surfactant, type IIIA includes oil, water soluble surfactant and Hydrophilic co-solvents, and type IV include surfactant and co-solvent mixture without oil. The main advantage is in these systems the drug remains in a dissolved state throughout its transit in the GIT. However, high amount of surfactants and chances of drug precipitation upon dilution with aqueous media are the major drawbacks of these systems [30].
Nanotechnology approaches: A variety of novel delivery systems are used to solubilize the lipophilic drugs, mainly classified as colloidal carrier systems (nanoemulsions, microemulsion,nanoparticles) and vesicular carrier systems (liposomes, transfersomes, ethosomes and niosomes). These carriers mainly enhance drug dissolution and permeability by their higher surface area and rendering the drug in the dissolved state. Solid lipid nanoparticles contain solid lipids as matrix material that possesses adhesive properties that make them adhere to the gut wall and release the drug exactly where it should be absorbed. Nanomorph is a recent technology converts the crystalline drug particles to amorphous nano-particles with higher dissolution rates [31].
Table 3: Cyclodextrin derivatives used in solubility enhancement: selected examples.

Inhibition of p-glycoprotein efflux
P-glycoprotein (P-gp) is one of the first members of the ATPbinding cassette (ABC) transporter can potentially reduce the absorption and oral bioavailability of a number of drugs [32]. P-gp inhibitor is usually co-administered with the drug to enhance drug absorption. Recently, many excipients are identified as P-gp inhibitors which including Surfactants and solubilizing solvents, Pluronic block copolymers, Lipid excipients and thiomers were identified as P-gp inhibitors [33]. Surfactants and lipids act indirectly and non-specifically by interacting with the lipid bilayer, increases membrane fluidity and permeability. Besides use of surfactants and lipids, many novel drug delivery carriers like dendrimers, nanoparticles and liposomes have P-gp eluding activity, since they are transported into the cells via receptor mediated endocytosis in contrast to the typical free drug diffusion. Research also shown that P-gp substrates such as propranolol encapsulated within dendrimer structures is an effective strategy to bypass the P-gp efflux pump [34,35].
Conclusion
The enhancement of solubility and improving bioavailability remains one of the most challenging aspects of drug development. There is need to identify the most suitable approach at early stages among the pool of different approaches. Each method has its own limitations. However different approaches aids the targeted delivery, sustained delivery and improves the pharmacokinetics profile, diffusion of drugs into various organs by crossing the barriers including the blood brain barrier. Further research can focus on these complexes and may lead to reduction of the doses of pharmaceuticals due to improved bioavailability.
References
- Ojha N, Prabhakar B (2013) Advances in solubility enhancement techniques. Int J Pharm Sci Rev Res 21(2): 351-358.
- Luque de Castro MD, Priego-Capote F (2007) Ultrasound-assisted crystallization (sonocrystallization). Ultrason Sonochem 14(6): 717-724.
- Dohrn R, Bertakis E, Behrend O, Voutsas E, Tassios D (2007) Melting point depression by using supercritical CO2 for a novel melt dispersion micronization process. J Mol Liq 131-132: 53-59.
- Won DH, Kim MS, Lee S, Park JS, Hwang SJ (2005) Improved physicochemical characteristics of felodipine solid dispersion particles by supercritical anti-solvent precipitation process. Int J Pharm 301(1-2): 199-208.
- Parikh RK, Mansuri NS, Gohel MC, Soniwala MM (2005) Indian drugs. Dissolution-Enhancement-of-Nimesulide-using-Complexation 42(3): 149-154.
- Borhani DW, Shaw DE (2012) The future of molecular dynamics simulations in drug discovery. J Comput Aided Mol De 26(1): 15-26.
- Shah JC, Chen JR, Chow D (1995) Preformulation study of etoposide: II Increased solubility and dissolution rate by solid-solid dispersions. Int J Pharm 113(1): 103-111.
- Ghebremeskel AN, Vemavarapu C, Lodaya M (2007) Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Selection of polymer–surfactant combinations using solubility parameters and testing the processability. Int J Pharm 328(2): 119-129.
- Paradkar A, Ambike AA, Jadhav BK, Mahadik KR (2004) Characterization of curcumin-PVP solid dispersion obtained by spray drying. Int J Pharm 271(1-2): 281-286.
- Doshi DH, Ravis WR, Betageri GV (1997) Carbamazepine and polyethylene glycol solid dispersions: preparation, in vitro dissolution, and characterization. Drug Dev Ind Pharm 23(12): 1167-1176.
- Liu Y, Wang T, Ding W, Dong C, Wang X, et al. (2018) Dissolution and oral bioavailability enhancement of praziquantel by solid dispersions. Drug Deliv Transl Res doi: 10.1007/s13346-018-0487-7.
- Hurley D, Potter CB, Walker GM, Higginbotham CL (2018) Investigation of ethylene oxide-co-propylene oxide for dissolution enhancement of hot-melt extruded solid dispersions. J Pharm Sci 3549(18): 30040- 30046.
- Vippagunta SR, Wang Z, Hornung S, Krill SL (2007) Factors affecting the formation of eutectic solid dispersions and their dissolution behavior. J Pharm Sci 96(2): 294-304.
- Aly AM (2008) Preparation of rapidly disintegrating glipizide tablets by surface solid dispersion through superdisintegrants. International Journal of Pharmaceutical Sciences and Nanotechnology 2008: 233-242.
- Chaturvedi M, Kumar M, Pathak K, Bhatt S, Saini V (2017) Surface solid dispersion and solid dispersion of meloxicam: comparison and product development. Adv Pharm Bull 7(4): 569-577.
- Loftsson T, Brewster ME (1996) Pharmaceutical applications of cyclodextrins 1 drug solubilization and stabilization. J Pharm Sci 85(10): 1017-1025.
- Pápay ZE, Sebestyén Z, Ludányi K, Kallai N, Balogh E, et al. (2016) Comparative evaluation of the effect of cyclodextrins and pH on aqueous solubility of apigenin. J Pharm Biomed Anal 117: 210-216.
- Avachat AM, Kulkarni JA, Avachat CM, Pradhan R, Suryawanshi TS, et al. (2017) Preferential formulation of second generation antipsychotic asenapine as inclusion complex with sulphobutylether-βcd (captisol): in vitro and in vivo evaluation. Curr Drug Deliv doi: 10.2174/1567201814 666171120121217.
- Kai-Hang L, Mengying S, Guping T, Xiurong HU (2017) Preparation, characterization and antitumor of cyclodextrin inclusion of an anti-cancer drug regorafenib. Zhejiang Da Xue Xue Bao Yi Xue Ban 46(2): 151-159.
- Lv P, Liu M, Liao R, Zhao Y, Liao X, et al. (2017) Host-guest inclusion system of rhein with polyamine-modified β-cyclodextrins: characterization and cytotoxicity. Pharm Dev Technol 22(5): 669-677.
- Mady FM, Farghaly Aly U (2017) Experimental, molecular docking investigations and bioavailability study on the inclusion complexes of finasteride and cyclodextrins. Drug Des Devel Ther 11: 1681-1692.
- Ates M, Kaynak MS, Sahin S (2016) Effect of permeability enhancers on paracellular permeability of acyclovir. J Pharm Pharmacol 68(6): 781- 790.
- Gidwani B, Vyas A (2015) Inclusion complexes of bendamustine with β-CD, HP-β-CD and Epi-β-CD: in-vitro and in-vivo evaluation. Drug Dev Ind Pharm 41(12): 1978-1988.
- Saleh A, McGarry K, Chaw CS, Elkordy AA (2018) Feasibility of using gluconolactone, trehalose and hydroxy-propyl gamma cyclodextrin to enhance bendroflumethiazide dissolution using lyophilisation and physical mixing techniques. Pharmaceutics 10(1).
- Fatmi S, Bournine L, Iguer-Ouada M, Lahiani-Skiba M, Bouchal F, et al. (2015) Amorphous solid dispersion studies of camptothecin-cyclodextrin inclusion complexes in PEG 6000. Acta Pol Pharm 72(1): 179-192.
- Londhe V, Shirsat R (2018) Formulation and characterization of fast-dissolving sublingual film of iloperidone using box-behnken design for enhancement of oral bioavailability. AAPS Pharm Sci Tech doi: 10.1208/ s12249-018-0954-y.
- Furuishi T, Takahashi S, Ogawa N, Gunji M, Nagase H, et al. (2017) Enhanced dissolution and skin permeation profiles of epalrestat with β-cyclodextrin derivatives using a cogrinding method. Eur J Pharm Sci 106: 79-86.
- Choi JM, Park K, Lee B, Jeong D, Dindulkar SD, et al. (2017) Solubility and bioavailability enhancement of ciprofloxacin by induced oval-shaped mono-6-deoxy-6-aminoethylamino-β-cyclodextrin. Carbohydr Polym 163: 118-128.
- Prandina A, Herfindal L, Radix S, Rongved P, Doskeland SO, et al. (2018) Enhancement of iodinin solubility by encapsulation into cyclodextrin nanoparticles. J Enzyme Inhib Med Chem 33(1): 370-375.
- Pouton CW (2000) Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and “self-microemulsifying” drug delivery systems. Eur J Pharm Sci 11(2): S93-S98.
- Kumar S, Dilbaghi N, Rani R, Bhanjana G, Umar A (2013) Novel approaches for enhancement of drug bioavailability. Rev Adv Sci Eng 2(2): 133-154.
- Lin JH, Yamazaki M (2003) Role of p-glycoprotein in pharmacokinetics. Clin Pharmacokinet 42(1): 59-98.
- Werle M, Hoffer M (2006) Glutathione and thiolated chitosan inhibit multidrug resistance P-glycoprotein activity in excised small intestine. J Control Release 111(1-2): 41-46.
- D’Emanuele A, Jevprasesphant R, Penny J, Attwood D (2004) The use of a dendrimer-propranolol prodrug to bypass efflux transporters and enhance oral bioavailability. J Control Release 95(3): 447-453.
- Svenson S (2009) Dendrimers as versatile platform in drug delivery applications. Eur J Pharm Biopharm 71(3): 445-462.
© 2018 Mirza R Baig. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






