- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Performance on Antibacterial Finishes for Textile Applications
Muhammad Naveed*
Department of Applied Chemistry, Government College University Faisalabad, Pakistan
*Corresponding author: Muhammad Naveed, Department of Applied Chemistry, Government College University Faisalabad, Allama Iqbal Road 38000, Pakistan
Submission: May 05, 2018; Published: May 24, 2018

ISSN 2578-0271 Volume2 Issue5
Abstract
Microorganisms are found everywhere in our world and most of them cause infectious diseases to human beings such as viruses, fungi, pathogenic bacteria etc. Suitable conditions like nutrients, warmth and humidity may accelerate the growth of these harmful microbes on textiles which not only increase the risk of spreading diseases but also cause some unwanted effects to textile products including loss of strength, decoloration and unpleasant smell. Antimicrobial agents are ultimate solution which inhibit and kill the microorganisms and are applied to textile products. Antimicrobial textiles are consumed continuously where fabric surface is expected to contact microorganisms. Much attentions have paid for development and finishing of these functional agents onto textiles. Various kinds of antimicrobial agents and finishes which provides superior safety, recharge ability, durability and excellent disinfection power are discussed in this article.
Keywords: Antimicrobial agents; Textile fabric; Antimicrobial activities; Mechanisms; Performances
Introduction
The discovery and applications of textile fabrics bearing antimicrobial functionalities has been one of most important and active research field in recent years. Antimicrobial finishes on textiles are anticipated to have resistance from spreading harmful microbes such as bacteria, fungi, spores, viruses etc. by killing them or inhibiting their growth. These finishes reduce the risk of spreading infectious diseases as well as minimize the hygienic issues. The antimicrobial finishes are mainly classified into biocidal and biostatic finishes. The textiles which can kill bacteria are called biocidal textiles while those which inhibit microbes are known as biostatic textiles. Odor control sportswear and conservation of textile artifacts require biostatic finishes but are not suitable to provide microbial protection for humans in their untreated form. Biostatic finishes are ultimate solution for human protections in medical applications which have ability to kill microbes rapidly as they contact the finished surface. Long term performance of antimicrobial finishes may achieve only by incorporation of limitless supply of agent in finish or customarily restore it on textiles, otherwise antimicrobial functionality will be lost on usage or exposure to air with the passage of time. Thus, durability of finish (especially storage and washing durability) on textile product is another challenge beside power of antimicrobial function. The practical methods for incorporation of antimicrobial agents include sol-gel technology, plasma treatments, microencapsulation, etc which could offer prolonged release of agents. Antimicrobial textiles should not cause irritation to skin and sensitization reactions when they have intimate contact with human skin. This article provides development, performances and progress of novel antimicrobial finishes on to textile fabrics.
Action of antibacterial agents
The choice of antimicrobial agent is always depend upon its final usage, requirements and way of its antimicrobial action. They act in two ways
(1) The antimicrobial agent chemically bind on the surface of treated fabric and do not detach from surface by its own. Microbes kill by agent when become in contact with treated fabric.
(2) The antimicrobial agent only physically attached with fibers and leaches or diffuses in environment and performs its action of killing microbes.
The former action losses the concentration of agent with the passage of time owing to its regular leaching and diffusion towards microbial environment and ultimately losses durability with usage and laundering. In later mechanism, although agent remain attached to fiber surface and do not leaches out but requires recharging of antimicrobial functionality as the reactive sites responsible for antimicrobial activity become consume during bacteria killing. Recently, third type of mechanism is observed to be adopted by nano-metal oxides which act as photocatalysts. They remain bind on surface of fiber and do not leach or diffuse out. Advantageously, they do not require recharging of antimicrobial functionalities. Actually, they utilize light energy to perform redox reaction with compounds in environment forming reactive oxygen species which further execute antibacterial action. In the whole mechanism, photoactalysts act as catalyst and do not lose its concentration and utilizes renewable energy source.
Antimicrobial agents
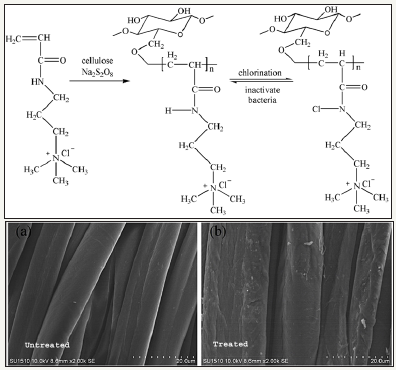
N-halamines: N-halamines are investigated as one of the most important antimicrobial agents possessing strong antimicrobial activity and safety, durability and rechargeability [1,2]. N-halamines are characterized by one or multiple polar nitrogen-halogen (N-X) covalent group which exhibit strong antimicrobial power and inactivate microbes through oxidative halogen. Depending upon chemical linkage, imide N-halamine, amide N-halamine and amine N-halamine are further three types of N-halamine. Their antimicrobial activities and stabilities vary due to presence of electron-withdrawing and electron-donating groups in environment of N-X bond. The N-X bond is reduced during killing microbes by N-halamine agents with an advantage of reversibility of antimicrobial function. Halogenating agent like dilute solution of bleaching solution can recover the biocidal activity as illustrated in Figure 1; [3,4]. This pronounced recharging of antimicrobial functionality separate them from other classes of biocidal agents. N-halamines attracted attention of researcher from both academic and industry after investigated them as water disinfectant with excellent water disinfectant abilities [5-8]. In this respect, 1,3-dimethylol-5,5-dimethly-hydantoin (DMDMH) based N-halamine was synthesized and utilized to fabricate antimicrobial textiles with rechargeable and durable functionalities [9]. DMDMH serves as crosslinker between N-halamine and fabric. However, this agent showed some decreased functionality during wearing [10]. Moreover, DMDMH releases formaldehyde which causes health hazards. Polycarboxylic acids were found good alternatives for crosslinking N-halamines with advantage of better water solubility and free from formaldehyde residues [11,12]. Later one, a large number of stable N-halamine agents were investigates which showed better performance during usage of finished textiles such as polymeric N-halamines [13-19], unsaturated N-halamines [20-23], reactive N-halamines [24-28], N-halamine based imidazolidones [29] and siloxane based N-halamines [30- 35]. A combination of N-halamines with other biocidal agents such as quaternary ammonium salts [36-41], titanium dioxide [42] and triclosan [43] are also found potential way regarding stability, durability and antimicrobial power. These coatings are applied both physically and chemically. However, N-halamine antimicrobial agents may also be incorporated prior to extrusion process for textile fiber production [44-47]. Synthesis route of antimicrobial cotton finished with N-halamine based methylene-bis-acrylamide is depicted in Figure 2.
Figure 1: Reduction of N-halamine during microbes killing and its recharging via bleaching.
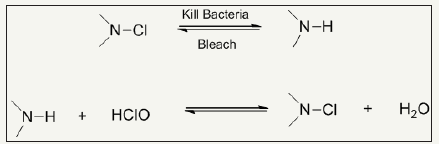
Figure 2: Synthesis route of antimicrobial cotton finished with N-halamine based methylene-bis-acrylamide [4].

Reactive N-halamines possess the reactive sites which are able to covalently attach on fibers surface. In this way, fabric provides durable antimicrobial functionality even after laundering. The chlorine loading lasts after 50 wash cycle which can be recover via bleaching. Examples are coated polyester [48] and cotton [24,25,49,50]. Polymeric N-halamines are such a antimicrobial polymers whose polymer back bone contains (i) one type of monomer exhibiting reactive group like epoxy ring which is able to covalently crosslinked onto cotton fabric and (ii) other monomer is a kind of N-halamine repeat unit capable of performing antimicrobial activity [12,17]. Figure 3 depicts the chemical structure of important epoxides based cyclic N-halamines. Hydroxyl and amine functionalized reactive reagents like 2,4,6-trichloros- triazine (chemical structure Figure 4) not only bound on cellulose fibers at low temperature but also prevents the fabric mechanical and physical damage under high temperature owing to its good thermal stability [27,51,52]. Moreover, N-halamines with higher hydrophilicity kill microbes more rapidly as compared to lower hydrophilic or strong hydrophobic ones [50]. A series of 2,4,6-trichloro-s-triazine based N-halamines has fabricated and investigated [26,53-55].
Figure 3: chemical structures of epoxides based cyclic N-halamine, (a) 1-glycidyl-s-triazine-2,4,6-trione and (b) 3-glycidyl-5,5-dialkylhydantoins.
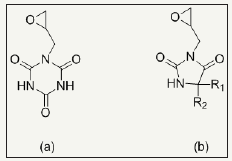
Figure 4: Chemical structure of hydroxyl and amine functionalized reactive reagent, 2,4,6-trichloro-s-triazine.
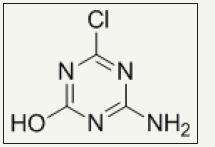
Figure 5: Chemical structures of (a) poly (3-(5,5-dimethylhydantoinylpropyl)siloxane-co-3 dimethyldodecylammonium propylsiloxane chloride) and (b) poly(1-chloro-5,5-dimethylhydantoinyl-3-ethyl-p-ethenylphenylmethyldimethylammonium chloride).
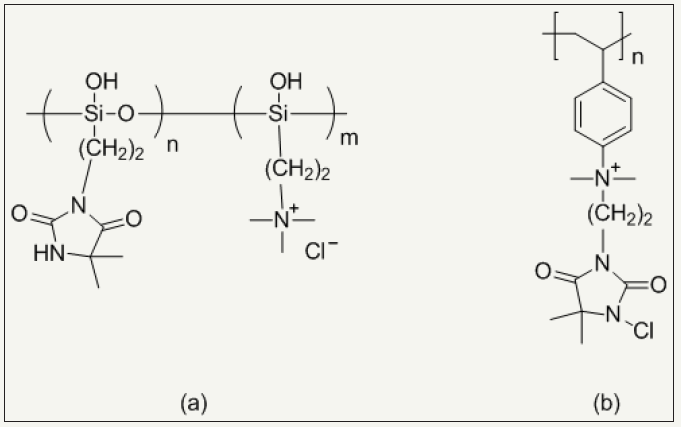
Another way to finish N-halamines on fiber surface is graft polymerization of fabric. Vinyl functionalized N-halamines can be grafted on fiber using free radical initiator. They are classified acyclic and cyclic N-halamines. Examples are 4-vinylbenzyl chloride [56], mechacrylate chloride [57], acryloyl chloride [58], hydroxyl ethylmethacrylate [19] and allyl bromide [20] based N-halamines. Quaternary ammonium salt based N-halamines further promote antimicrobial activity of N-halamines and exhibit excellent water solubility and ultimately ease of their application during fabric finishing [37,59,60]. Figure 5 shows the chemical structures of quaternary ammonium salt based N-halamines while Figure 6 show schematic of grafted cotton by quaternary ammonium salt based N-halamines.
Figure 6: schematic of grafted cotton by quaternary ammonium salt based N-halamines and their scanning electron microscopy (SEM) images [59].
Halogenated phenols: Halogenation of phenols imparts improved antibacterial functionality to phenols. Para halogenated phenols have greater potential as compared to other halogenated phenols, however, with decreased water solubility. A number of halogenated products of phenols are depicted in Figure 7. Triclosan is most important bactericidal among halogenated phenols and exhibit good antimicrobial power against a broad spectrum of microbes and antibiotic-resistant bacteria. It is used in antibacterial household textiles, cleaning wipes, towels, socks, schoe-socks, for proectection of transport and industrial filter [61-63]. Triclosan block the lipid biosynthesis of microbes and ultimately inhibit the fatty acid biosynthesis. Beside this, it also inhibits growth of microbes by reacting with amino-acids of active enzyme’s sites [64]. Halogenated phenols are not able to form strong bond with fiber surface, so they perform their activity through leaching mechanism. 6% Triclosan solution applied to cotton fabric retain its antibacterial up to 50 laundering washing cycles [64]. The agent leaches slowly and kills microbes in its environment [65]. However, these agents lose their concentration on usage with passage of time and become effective less. Recently, new strategies are investigated where halogenated phenolic antimicrobial agents are tried to bind on the fiber surface chemical by using suitable cross linkers. This method is however increasing the durability of antimicrobial functionality of halogenated phenols. The binding mechanism of triclosan on cellulose fiber is illustrated in Figure 8.
Figure 7: Chemical structures of various halogenated products of phenols
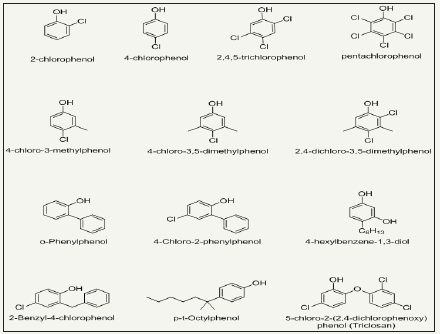
Figure 8: Binding mechanism of triclosan on to cellulose fiber using diacid a crosslinker [66].
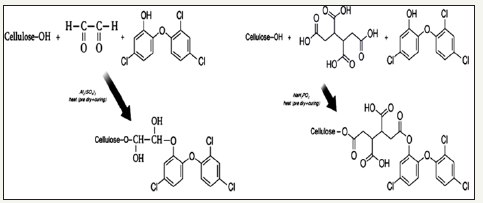
Figure 9: Chemical structure of polybiguanide from hexamethylene biguanide monomer, repeating using n=15-20, Molecular weight ~ 3000.
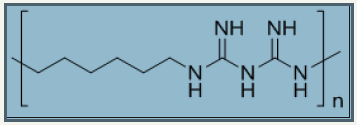
Polybiguanides: Polybiguanides are water soluble polymers of repeating unit alkyl biguanides. Polyhexamethylene biguanides are polymer of “hexamethylene biguanides” (HMB) repeat unit with degree of polymerization [15-20]. Figure 9 presents the chemical structure of PHMB. Polybiguanides are specially utilized as cleansing agents in food industry with 20% aqueous solution [66]. Their concentration between 5-25mg/ml is required to inhibition of bacteria but a concentration up to 250mg/ml may also require some bacteria like Pseudomonas vulgaris and P.aeruginosa. PHMB is expected to inhibit gram positive bacteria in a way by which it activate outer membrane of lipopolysaccharide which then displaces cation via self-promotion mechanism [67-71]. Polybiguanides not only possess good antimicrobial activity but also have strong affinity to attach on surface of cotton fabric. The electrostatic interactions between negatively charged bacteria cell wall and positively charged biguanide repeat unit are responsible to kill bacteria. Advantageously, positively charged biguanide groups are able to bind with negatively charged group’s especially carboxylic group in cellulosic fabrics via electrostatic interactions [72]. Moreover, its applicability becomes easier in exhaustion and padding processes due to its water soluble nature.
Polybiguanides do not leach out and remain chemically bound to fiber surface where they act as barrier against microbes which come in contact with treated fiber and show good durability [72,73].
Natural products as antimicrobial agents
Natural products from secondary metabolites of plants have well known to human beings for cure of human diseases as they exhibit various biological activities on other organisms. These natural products are now also utilizing in commercial drugs and medicines. They also are being consumed in beverages, foods, flavoring, dyes, fibers and fragrances. Owing to their low toxicity, biocompatibility and biological activities, they have raised the interest of textiles engineers to utilize them for fabrication various functional textiles. They are investigated for evaluating various properties for textile products such as insecticidal effects, antimicrobial effects, vitalizing and energizing effects, promotion of comfort, health and fitness, UV protection and pleasant odor.
Synthetic antimicrobial agent although show good antibacterial power and durability, but toxic nature of some synthetic agents and their non-degradability in environment has shifted the trend of their utilization towards natural products as competitive alternatives. A small fraction of natural products have studied among 500000 known plant species.
Figure 10:Chemical structures of some important natural antibacterial agents for antimicrobial finish of textiles.
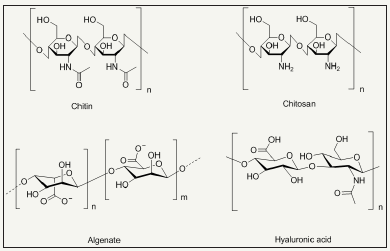
The investigated natural product based antimicrobial agents for textile finishing are phenols, polyphenols, quinones, flavonoids, tannins, coumarins, terpenoids, natural polymers like chitin, chitosan, alginate, gelatin and hyaluronic acid [74-83]. The chemical structures of some of natural products are depicted in Figure 10.
Photoactive antimicrobial agents
Figure 11: Chemical structures of some organic photosensitizers
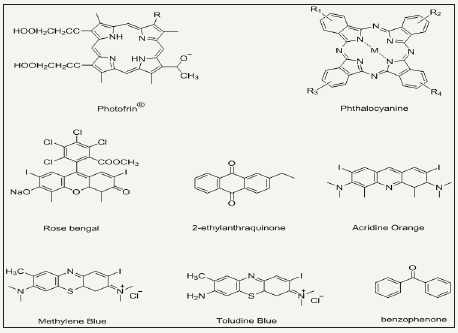
Figure 12: schematic redox mechanism of photocatalyst via photoexcitation and de-excitation producing reactive oxygen species by performing redox reaction with compounds in its environment, highly reactive oxygen species have potential to kill a broad spectrum of microorganism non-selectively with the advantage of good durability.
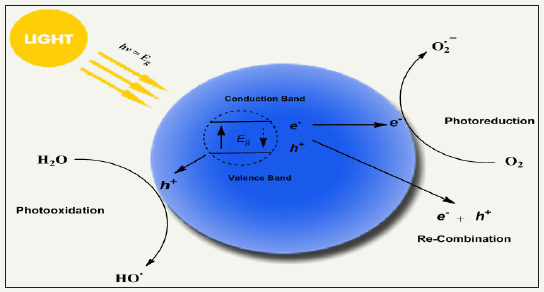
Photo-antimicrobial agents are light activated antimicrobial agents which trigger their antimicrobial activity upon light irradiation and cease in absence of light. The microbial resistance of light activated antimicrobial agents is purely dependent on exposure time, irradiation distance, light intensity and light source as well. Photoactive antimicrobial agents may either organic photosensitizer dyes or inorganic photocatalysts. Organic photosensitizers may be synthetic or natural such as phenothiazinium, rose Bengal, porphyrins etc (Figure 11). Inorganic photocatalysts are mostly metal oxide nanoparticles (NPs), for example, TiO2, ZnS, ZnO, Fe2O3 and SnO2 based semiconductors [84-95]. Actually, a photochemical reaction occur when these agents absorb light and as a result they continuously emit reactive oxygen species under light irradiation in visible (350-700nm) or UVA range. The generated reactive oxygen species may be hydrogen peroxide (H2O2), hydroxyl radical (HO), singlet oxygen (1O2) etc. Such species are able to inactivate a broad range of microbes including Pseudomonas aeruginosa Candida albicans, C. difficile, Escherichia coli, S. aureus, etc. Photoactive antimicrobial agent possess excellent antimicrobial activities under light irradiation with durability. There is no need to supply agents regularly as those halogenated phenols require. They remain attach on fiber surface, however, wearing may cause the loss of agent from fabric surface. The schematic redox mechanism via photoexcitation and de-excitation is presented in Figure 12. Photocatalysts possess band structure rather than continuous electronic states like metals. Excitation of electron from valence band to conduction band occur which is assisted by absorption of photons from light source (hν>ΔEg) [96,97]. Such excitation result in formation of holeelectron pair where a hole (h+) bearing positive charge produce in valence band and active electron (e-) produce in conduction band. Here, there may two chances of happenings. One is that the excited electron may come back to valence bond by releasing heat. Secondly, here in excited state, a redox reaction may happen with absorbed compounds from nearby environment, for example, molecular oxygen, water or hydroxyl groups [98]. As a result of such redox reactions, various reactive oxygen species formed depending upon the absorbed compound. These extremely active oxygen species then kill the bacterial non-selectively and perform their strong antibacterial activity. Most interestingly, photoactive antimicrobial agents act as catalyst and eventually go back to its original form via excitation/de-excitation process during redox reaction and impart durability to treated fabric. Thus photocatalysts do not lose their concentrations, no need to recharging of functionality, and thus ultimately they present excellent durability and antimicrobial activity.
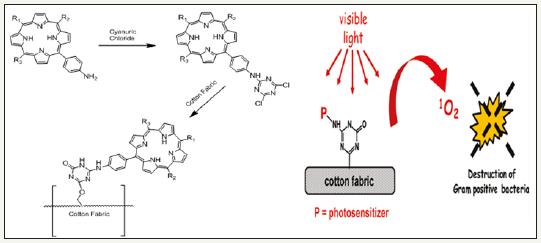
Organic photosensitizers go in their excited state by absorbing particular amount of energy and produce reactive oxygen species via two photochemical methods [99-103]. These mechanisms are illustrated in Figure 13. The synthesis route and bacterial resistance of photo-antimicrobial cotton is presented in Figure 14.
Figure 13: Mechanism of producing reactive oxygen species of photosensitizers via photosensitization process.
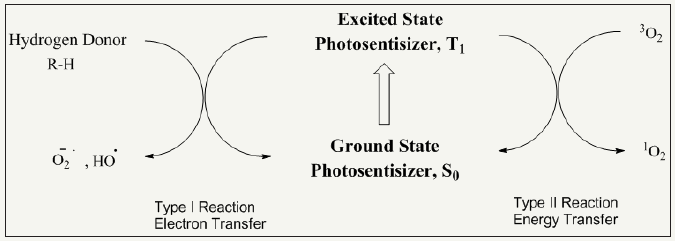
Figure 14: Synthesis route and bacterial resistance of photo-antimicrobial cotton [103].
Conclusion
Antimicrobial finishes are applied to textile products for a number of advantage regarding human health issures such as, inhibiting microbes from spreading, control of infections, control of odor, healing of wound, etc. antimicrobial textile should meet a number of key requirements including excellent resistance towards microbes, durability, should be safe to human skinand body. antimicrobial acitvities, durability, mechanisms and performces of various classes of antimicrobial agents is dicussed. The major classes of antimicrobial agents for textile finishing applications are N-halamines, halogenated phenols, polybiguanides, plant based natural products, and photoactive animicrobial agents. In future, development of antimicrobial finishes with precised long term antimicrobial power, durability and actual wearing and laundring conditions should be practice out.
References
- Chen Z, Luo J, Sun Y (2007) Biocidal efficacy, biofilm-controlling function, and controlled release effect of chloromelamine based bioresponsive fibrous materials. Biomaterials 28(9): 1597-1609.
- Akdag A, Okur S, McKee ML, Worley SD (2006) The stabilities of N-Cl bonds in biocidal materials. J Chem Theory Comput 2(3): 879-884.
- Liu Y, Ren X, Liang J (2015) Antibacterial modification of cellulosic materials. Bio Resources 10(1): 1964-1985.
- Tian H, Zhai Y, Xu C, Liang J (2017) Durable antibacterial cotton fabrics containing stable acyclic N-Halamine groups. Ind Eng Chem Res 56(28): 7902-7909.
- Sun G, Wheatley WB, Worley SD (1994) A new cyclic N-Halamine biocidal polymer. Ind Eng Chem Res 33(1): 168-170.
- Sun G, Allen LC, Luckie EP, Wheatley WB, Worley SD (1995) Disinfection of water by N-Halamine biocidal polymers. Ind Eng Chem Res 34(11): 4106-4109.
- Sun G, Chen TY, Worley SD (1996) A novel biocidal styrenetriazinedione polymer. Polymer (Guildf) 37(16): 3753-3756.
- Tsao TC, Williams DE, Worley CG, Worley SD (1991) Novel N‐Halamine disinfectant compounds. Biotechnol Prog 7(1): 60-66.
- Sun Y, Sun G (2001) Novel regenerable N-halamine polymeric biocides. II. Grafting hydantoin-containing monomers onto cotton cellulose. J Appl Polym Sci 81(3): 617-624.
- Ren X, Kocer HB, Worley SD, Broughton RM, Huang TS (2009) Rechargeable biocidal cellulose: Synthesis and application of 3-(2, 3-dihydroxypropyl)-5, 5-dimethylimidazolidine-2, 4-dione. Carbohydrate Polymer 75(4): 683-687.
- Lee J, Broughton RM, Akdag A, Worley SD, Huang TS (2007) Antimicrobial fibers created via polycarboxylic acid durable press finishing. Text Res J 77(8): 604-611.
- Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS (2012) Multifunctional cotton fabric: Antimicrobial and durable press. J Appl Polym Sci 124(5): 4230-4238.
- Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS (2011) N-halamine biocidal coatings via a layer-by-layer assembly technique. Langmuir 27(7): 4091-4097.
- Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS (2012) N-halamine copolymers for biocidal coatings. React Funct Polym 72(10): 673-679.
- Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS (2013) Antimicrobial surface coatings for polypropylene nonwoven fabrics. React Funct Polym 73(11): 1412-1419.
- Cerkez I, Kocer HB, Worley SD, Broughton RM, Huang TS (2013) Antimicrobial coatings for polyester and polyester/cotton blends. Prog Org Coatings 76(7-8): 1082-1087.
- Kocer HB, Cerkez I, Worley SD, Broughton RM, Huang TS (2011) Polymeric antimicrobial N-halamine epoxides. ACS Appl Mater Interfaces 3(8): 2845-2850.
- Liu Y, Li J, Cheng X, Ren X, Huang TS (2015) Self-assembled antibacterial coating by N- halamine polyelectrolytes on a cellulose substrate. J Mater Chem B Mater Biol Med 3: 1446-1454.
- Chang D, li Z, Wang X, Zhu C, Dong A, et al. (2018) N-Halamine polymer from bipolymer to amphiphilic terpolymer with enhancement in antibacterial activity. Colloids Surf B Biointerfaces 163: 402-411.
- Sun Y, Sun G (2002) Durable and regenerable antimicrobial textile materials prepared by a continuous grafting process. Journal of Applied Polymer Science 84(8): 1592-1599.
- Luo J, Sun Y (2008) Acyclic N-halamine coated kevlar fabric materials: Preparation and biocidal functions. Industrial Engineering Chemistry Research 47(15): 5291-5297.
- Sun Y, Sun G (2001) Novel regenerable N-halamine polymeric biocides. III. Grafting hydantoin-containing monomers onto synthetic fabrics. Journal of Applied Polymers Science 81(6): 1517-1525.
- Ren X (2008) Antimicrobial coating of an N-halamine biocidal monomer on cotton fibers via admicellar polymerization. Colloids Surfaces A Physicochem Eng Asp 317(1-3): 711-716.
- Liang J (2007) Fabric treated with antimicrobial N-halamine epoxides. Industrial Engineering Chemistry Research 46(20): 6425-6429.
- Ma K (2013) Synthesis of novel N -halamine epoxide based on cyanuric acid and its application for antimicrobial finishing. Industrial Engineering Chemistry Research 52(22): 7413-7418.
- Gouda M, Ibrahim NA (2008) New approach for improving antibacterial functions of cotton fabric. Journal of Indostrial Textile 37(4): 327-339.
- Sun Y, Chen Z, Braun M (2005) Preparation and physical and antimicrobial properties of a cellulose-supported chloromelamine derivative. Industrial Engineering Chemistry Research 44(21): 7916- 7920.
- Cerkez I, Kocer HB, WorleySD, Broughton RM, Huang TS (2016) Antimicrobial functionalization of poly(ethylene terephthalate) fabrics with waterborne N-halamine epoxides. Journal of Applied Polymer Science 133(9).
- Ren T, Qiao M, Huang TS, Weese J, Ren X (2018) Efficacy of N-halamine compound on reduction of microorganisms in absorbent food pads of raw beef. Food Control 84: 255-262.
- Liang J (2007) Improved antimicrobial siloxane. Industrial Engineering Chemistry Research 46(7): 1861-1866.
- Chen Y, Zhong XS, Zhang Q (2012) Synthesis of CO2-philic polysiloxane with N-halamine side groups for biocidal coating on cotton. Industrial Engineering Chemistry Research 51(27): 9260-9265.
- Kocer KB, Akdag A, Ren X, Broughton RM, Worley SD, et al. (2008) Effect of alkyl derivatization on several properties of N-halamine antimicrobial siloxane coatings. Industrial Engineering Chemistry Research 47(20): 7558-7563.
- Kocer HB, Worley SD, Broughton RM, Acevedo O, Huang TS (2010) Effect of phenyl derivatization on the stabilities of antimicrobial N -chlorohydantoin derivatives. Industrial Engineering Chemistry Research 49(22): 11188-11194.
- Chen Y, Yu P, Feng C, Wang Y, Han Q, et al. (2017) Synthesis of polysiloxane with quaternized N-halamine moieties for antibacterial coating of polypropylene via supercritical impregnation technique. Applied Surface Science 419: 683-691.
- Jiang Z, Demir B, Broughton RM, Ren X, Huang TS, et al. (2016) Antimicrobial silica and sand particles functionalized with an N-halamine acrylamidesiloxane copolymer. Journal of Applied Polymer Science 133(19).
- Kou L (2009) Synthesis of a water-soluble siloxane copolymer and its application for antimicrobial coatings. Industrial Engineering Chemistry Research 48(14): 6521-6526.
- Liang J, Chen Y, Barnes K, Wu R, Worley SD, et al. (2006) N-halamine/ quat siloxane copolymers for use in biocidal coatings. Biomaterials 27(11): 2495-2501.
- Kang ZZ, Zhang B, Jiao YC, Xu YH, He QZ, et al. (2013) High-efficacy antimicrobial cellulose grafted by a novel quaternarized N-halamine. Cellulose 20(2): 885-893.
- Zhang B, Jiao Y, Kang Z, Ma K, Ren X, et al. (2013) Durable antimicrobial cotton fabrics containing stable quaternarized N-halamine groups. Cellulose 20(6): 3067-3077
- Hu B, Chen X, Zuo Y, Liu Z, Xing X (2014) Dual action bactericides: Quaternary ammonium/N-halamine-functionalized cellulose fiber. Journal of Applied Polymer Science 131(7).
- Chen Y, Yu P, Ren G, Zhang Q, Han Q, Teng H (2017) Interpenetration of polyethylene terephthalate with biocidal quaternary ammonium/NChloramine polysiloxane in supercritical CO2. Industrial Engineering Chemistry Research 56(34): 9560-9568.
- Li J, Li R, Du J, Ren X, Worley SD, et al. (2013) Improved UV stability of antibacterial coatings with N-halamine/TiO2. Cellulose 20(4): 2151- 2161.
- Jiang Z, Fang L, Ren X, Huang TS (2015) Antimicrobial modification of cotton by reactive triclosan derivative. Fibers and Polymers 16(1): 31- 37.
- Li R (2014) Preparation and antimicrobial activity of β-cyclodextrin derivative copolymers/cellulose acetate nanofibers. Chemical Engineering Journal 248: 264-272.
- Sun X (2010) Electrospun composite nanofiber fabrics containing uniformly dispersed antimicrobial agents as an innovative type of polymeric materials with superior antimicrobial efficacy. ACS Appl Mater Interfaces 2(4): 952-956.
- Ren X, Akdag A, Zhu C, Kou L, Worley SD, et al. (2009) Electrospun polyacrylonitrile nanofibrous biomaterials. Journal of Biomedical Material Research-Part A 91(2): 385-390.
- Jiang Q, Jiang Z, Ma K, Li R, Du J, et al. (2014) Development of cytocompatible antibacterial electro-spun nanofibrous composites. Journal of Materials Science 49(19): 6734-6741.
- Ma K, Jiang Z, Li L, Liu Y, Ren X, et al. (2014) N-halamine modified polyester fabrics: Preparation and biocidal functions. Fibers and Polymers 15(11): 2340-2344.
- Jiang Z, Peng W, Li R, Huang D, Ren X, et al. (2017) Synthesis and application to cellulose of reactive dye precursor of anti-bacterial N-halamine. Coloration Technology 133(5): 376-381.
- Jiang Z, Ma K, Du J, Li R, Ren X, et al. (2014) Synthesis of novel reactive N-halamine precursors and application in antimicrobial cellulose. Applied Surface Science 288: 518-523.
- Braun M, Frank W, Ganter C (2011) First structurally characterized triazinyl palladium complexes via oxidative addition of cyanuric chloride derivatives. Journal of Organometallic Chemistry 696(22): 3580-3583.
- Braun M, Sun Y (2004) Antimicrobial polymers containing melamine derivatives. I. Preparation and characterization of chloromelaminebased cellulose. Journal of Polymer Science Part A: Polymer Chemistry 42(15): 3818-3827.
- Lee J, Broughton RM, Akdag A, Worley SD, Huang TS (2007) Preparation and application of an s-Triazine-based novel N-halamine biocide for antimicrobial fibers. Fibers and Polymers 8(2): 148-154.
- Jiang Z, Qiao M, Ren X, Zhu P, Huang TS (2017) Preparation of antibacterial cellulose with s-triazine-based quaternarized N-halamine. Journal of Applied Polymer Science 134(26).
- Jiang Z, Liu Y, Li R, Ren X, Huang TS (2016) Preparation of antibacterial cellulose with a monochloro-s-triazine-based N-halamine biocide. Polymer Advanced Technologiees 27(4): 460-465.
- Ren X (2009) Antimicrobial modification of polyester by admicellar polymerization. J Biomed Mater Res B Appl Biomater 89(2): 475-480.
- Yao J, Sun Y (2008) Preparation and characterization of polymerizable hindered amine-based antimicrobial fibrous materials. Industrial Engineering Chemistry Research 47(16): 5819-5824.
- Liu Y, Jiang Z, Li J, Liu Y, Ren X, et al. (2015) Antibacterial functionalization of cotton fabrics by electric-beam irradiation. Journal of Applied Polymer Science 132(23).
- Liu Y, Ren X, Huang TS (2014) Antimicrobial cotton containing N-halamine and quaternary ammonium groups by grafting copolymerization. Appl Surf Sci 296: 231-236.
- Liu Y, Ma K, Li R, Ren X, Huang TS (2013) Antibacterial cotton treated with N-halamine and quaternary ammonium salt. Cellulose 20(6): 3123- 3130.
- Shanmugasundaram OL (2007) Recent developments in medical textiles. J Text Assoc 68(4): 193-197.
- Rajendran S, Anand SC (2002) Developments in medical textiles. Text Prog 32(4): 1-42.
- Welk A (2005) The effect of a polyhexamethylene biguanide mouthrinse compared with a triclosan rinse and a chlorhexidine rinse on bacterial counts and 4-day plaque re-growth. J Clin Periodontol 32(5): 499-505.
- Orhan M, Kut D, Gunesoglu C (2007) Use of triclosan as antibacterial agent in textiles. Indian Journal of Fibre & Textile Research 32(1): 114- 118.
- Kut D, Orhan M, Günesoglu C, Özakin C (2005) Effects of environmental conditions on the antibacterial activity of treated cotton knits. AATCC Rev 5(3).
- Novikov M, Thong KL, Zazali NIM, Hamid SBA (2018) Treatment of cotton by β-Cyclodextrin/triclosan inclusion complex and factors affecting antimicrobial properties. Fibers and Polymers 19(3): 548-560.
- Gilbert P, Pemberton D, Wilkinson DE (1990) Barrier properties of the gram-negative cell envelope towards high molecular weight polyhexamethylene biguanides. J Appl Bacteriol 69(4): 585-592.
- Broxton P, Woodcock PM, Gilbert P (1983) A study of the antibacterial activity of some polyhexamethylene biguanides towards Escherichia coli ATCC 8739. J Appl Bacteriol 54(3): 345-353.
- Broxton P, Woodcock PM, Heatley F, Gilbert P (1984) Interaction of some polyhexamethylene biguanides and membrane phospholipids in Escherichia coli. J Appl Bacteriol 57(1): 115-124.
- Broxton P, Woodcock PM, Gilbert P (1984) Binding of some polyhexamethylene biguanides to the cell envelope of Escherichia coli ATCC 8739. Microbios 41(163): 15-22.
- Woodcock PM (1988) Biguanides as industrial biocides. Ind biocides, pp. 9-36.
- Simoncic B, Tomsic B (2010) Structures of novel antimicrobial agents for textiles-A review. Textile Research Journal 80(16): 1721-1737.
- Schindler WD, Hauser PJ (2004) Chemical finishing of textiles
- Ghaheh FS, Mortazavi SM, Alihosseini F, Fassihi A, Nateri AS, et al. (2014) Assessment of antibacterial activity of wool fabrics dyed with natural dyes. Journal of Cleaner Production 72: 139-145.
- Zahran MK, Ahmed HB, El-Rafie MH (2014) Surface modification of cotton fabrics for antibacterial application by coating with AgNPsalginate composite. Carbohydrate Polymers 108: 145-152.
- Varaprasad K, Raghavendra GM, Jayaramudu T, Seo J (2016) Nano zinc oxide-sodium alginate antibacterial cellulose fibres. Carbohydrate Polymers 135: 349-355.
- Su CH, Kumar GV, Adhikary S, Velusamy P, Pandian K, et al. (2017) Preparation of cotton fabric using sodium alginate-coated nanoparticles to protect against nosocomial pathogens. Biochemical Engineering Journal 117: 28-35.
- Lin J (2018) Durably antibacterial and bacterially antiadhesive cotton fabrics coated by cationic fluorinated polymers. ACS Appl Mater Interfaces 10(7): 6124-6136.
- Bhuiyan MAR, Islam A, Islam S, Hossain A, Nahar K (2017) Improving dyeability and antibacterial activity of Lawsonia inermis L on jute fabrics by chitosan pretreatment. Textile and Clothing Sustainability 3: 1.
- Zhang W, Dai X, Zhou J, Zhu G (2017) Antibacterial bombyx mori silk fabric modified with reactive chitosan quaternary ammonium salt and its laundering durability. Fibers and Polymers 18(2): 290-295.
- Han S, Yang Y (2005) Antimicrobial activity of wool fabric treated with curcumin. Dye Pigment 64(2): 157-161.
- Ghoreishian SM, Maleknia L, Mirzapour H, Norouzi M (2013) Antibacterial properties and color fastness of silk fabric dyed with turmeric extract. Fibers and Polymers 14(2): 201-207.
- Prabhu KH, Teli MD (2014) Eco-dyeing using Tamarindus indica L. seed coat tannin as a natural mordant for textiles with antibacterial activity. Journal of Saudi Chemical Society 18(6): 864-872.
- Banerjee S, Dionysiou DD, Pillai SC (2015) Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Applied Catalysis B: Environmental 176: 396-428.
- Tung WS, Daoud WA (2011) Self-cleaning fibers via nanotechnology: a virtual reality. Journal of Material Chemistry 21(22): 7858-7869.
- Radetić M (2013) Functionalization of textile materials with TiO2 nanoparticles. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 16: 62-76.
- Li S, Zhu T, Huang J, Guo Q, Chen G, et al. (2017) Durable antibacterial and UV-protective Ag/TiO2@ fabrics for sustainable biomedical application. Int J Nanomedicine 12: 2593-2606.
- Moqeet HA, Ahmed M, Afzal A, Jabbar A, Faheem S (2018) Characterization and antibacterial property of kapok fibers treated with chitosan/AgCl- TiO2 colloid. Journal of Textile Institute, pp. 1-5.
- Mishra A, Butola BS (2017) Deposition of Ag doped TiO2on cotton fabric for wash durable UV protective and antibacterial properties at very low silver concentration. Cellulose 24(8): 3555-3571.
- El-Naggar ME, Shaheen TI, Zaghloul S, El-Rafie MH, Hebeish A (2016) Antibacterial activities and UV protection of the in situ synthesized titanium oxide nanoparticles on cotton fabrics. Industrial & Engineering Chemistry Research 55(10): 2661-2668.
- Yadav HM, Kim JS, Pawar SH (2016) Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean Journal of Chemical Engineering 33(7): 1989-1998.
- Shaban M, Mohamed F, Abdallah S (2018) Production and characterization of superhydrophobic and antibacterial coated fabrics utilizing ZnO nanocatalyst. Scientific Reports 8(1): 3925.
- DÁgua RB (2018) Efficient coverage of ZnO nanoparticles on cotton fibres for antibacterial finishing using a rapid and low cost in situ synthesis. New J Chem 42(2): 1052-1060.
- Manna J, Begum G, Kumar KP, Misra S, Rana RK (2013) Enabling antibacterial coating via bioinspired mineralization of nanostructured ZnO on fabrics under mild conditions. ACS Appl Mater Interfaces 5(10): 4457-4463.
- Nakashima T, Sakagami Y, Matsuo M (2001) Antibacterial efficacy of cotton fabrics chemically modified by metal salts. Biocontrol Science 6(1):9-15.
- Mills A, Le Hunte S (1997) An overview of semiconductor photocatalysis. Journal of Photochemistry Photobiology A: Chem 108(1): 1-35.
- Rajh T, Makarova OV, Thurnauer MC, Cropek P (2000) Synthesis functionalization and surface treatment of nanoparticles. Am Sci Publ, pp. 147-171.
- Ireland JC, Klostermann P, Rice EW, Clark RM (1993) Inactivation of Escherichia coli by titanium dioxide photocatalytic oxidation. Applied and Environmental Microbiology 59(5): 1668-1670.
- Wilson BC, Patterson MS (2008) The physics, biophysics and technology of photodynamic therapy. Phys Med Biol 53(9): R61-109.
- Robertson CA, Evans DH, Abrahamse H (2009) Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B Biol 96(1): 1-8.
- Castriciano MA (2017) Poly (carboxylic acid)-Cyclodextrin/ Anionic porphyrin finished fabrics as photosensitizer releasers for antimicrobial photodynamic therapy. Biomacromolecules 18(4): 1134-1144.
- Özdemir PS, Özgüney AT, Kantar GK, \cSa\csmaz S, (2017) Effect of eugenol group and central metal ion on fastness and antibacterial properties of metallophthalocyanines printed on cotton fabric. AATCC Journal of Research 4(3): 34-45.
- Ringot C (2011) Triazinyl porphyrin-based photoactive cotton fabrics: Preparation, characterization, and antibacterial activity. Biomacromolecules 12(5): 1716-1723.
© 2018 Muhammad Naveed. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






