- Submissions

Full Text
Techniques in Neurosurgery & Neurology
Early Quinidine Treatment in Migrating Focal Seizures with Kcnt1 Mutation
Cristina Cáceres-Marzal1*, Dolores Sardina1 and Máximo Guillén-Sánchez2
1Paediatric Neurology Unit, University Children Hospital Badajoz, Spain
1Neurophysiology Unit, university Children Hospital Badajoz, Spain
*Corresponding author:Cristina Cáceres Marzal, Paediatric Neurology Unit, University Children Hospital Badajoz, Spain
Submission: December 06, 2023;Published: March 20, 2024

ISSN 2637-7748
Volume5 Issue5
Abstract
Background: Epilepsy of Infancy with Migrating Focal Seizures (EIMFS) is an early epileptic
encephalopathy with a poor prognosis. Patient description: The patient is a 3 years-old male who
presented focal and migrating seizures since the first months of life. Serial Video-Electroencephalogram
(video-EEG) showed a progressive slowing of background activity and multifocal discharges. Numerous
anticonvulsant drugs were tested without success. Whole Exome Sequencing (WES) revealed a
heterozygous, de novo, missense mutation in KCNT1 (c. 1429G>A; p.Ala477Thr). At seven months old
quinidine treatment was beginning. At that time, he had more than 20 seizures per day. Progressively the
dose was increased according to plasma levels. After two months he had 2-4 seizures per day and showed
a slight improvement in his social and motor development.
Conclusion: EIMFS is a devastating condition to lead to an encephalopathic syndrome in a large
majority of cases. Seizure control, particularly in early phases of the syndrome, may improve outcomes.
The onset of quinidine treatment in early stages of development helps to improve seizures control and
neurodevelopmental disabilities
Introduction
Epilepsy of Infancy with Migrating Focal Seizures (EIMFS) is an early epileptic encephalopathy characterized by multifocal seizures with migration of ictal focal discharges and arrest of psychomotor development. Seizures become very frequent, occurring in cluster and often evolves into near-continuous seizures or status epilepticus. Sometimes seizures may be precipitated by intercurrent diseases [1]. The syndrome usually lead to a poor prognosis in both seizures control and neurodevelopmental outcomes. Multiples genes have been implicated in EIMFS etiology to date, including PLCB1, SCN2A, SCN1A, TBC1D24, SLC25A22, SCN8A, SLC12A5, GABRB3, KCNT1 and KCNT2 [2]. Mutations in KCNT1 appear to be the most commonly known genetic cause of EIMFS [3,4]. KCNT1 encodes a sodium-activated potassium channel that is widely expressed in both neurons and cardiomyocytes [5]. Its activity modulates the hyperpolarization after repetitive firing of action potentials in neurons, and its C-terminal domain interacts with FMRP (Fragile-X Mental Retardation Protein) [3]. Gain-of-function mutations of this gene leads to a hyperactivation of the channel, generating a prolonged hyperpolarization, resulting in an imbalance between neuronal excitation and inhibition that lead to seizures [6,7]. The consequences of the KCNT1 mutations appear more severe than those reported for other potassium channel [3], which indicates that may also be other mechanisms independently of ion flux that leading to severe neurodevelopmental delay [7]. KCNT1 mutations have been also found in patients affected with autosomal dominant nocturnal frontal epilepsy, Ohta hara syndrome, multifocal epilepsy and west syndrome [3,4]. Recently, have been associated others epileptic syndromes to KCNT1 mutations, including mesial temporal epilepsy, cerebellar ataxia and intellectual disability [8,9]. Most of patients with KCNT1 mutations reported had intractable seizures that were only temporarily controlled with antiepileptic drugs [4]. Since KCNT1 is not affected by any conventional anti convulsive ants, exists an urgent need for novel therapies.
Patient Description
The patient is a now 3-year-old male who was born at term of non-consanguineous parents. There was no family history of neurologic disorders. For about first 24 hours of life, he presented shorts and focal motor seizures. It is likely that the beginning of the seizures was during the foetal stage, since the mother could appreciate foetal jerks during the last gestational-weeks. There was initially a good responsive to phenobarbital therapy. Laboratory exams, including infectious and extensive metabolic diseases testing’s, were normal. Neuroimaging exams were normal too. Initial EEG showed little central discharges. General physical and neurologic examination was normal. A few weeks later seizures reappear more markedly, characterized by brief episodes of eye deviation with head turning, unilateral motor activity, generalized tonic stiffening and autonomic symptoms. Seizures became very frequent, occurring in cluster or being almost continuous for days. Serial video-EEG showed a progressive slowing of background activity and increase of the interictal multifocal discharges. Subclinical and clinical ictal EEG patterns emaning mainly from right temporal and posterior head regions, ranging seizure semiology according to the focal origin. Numerous anticonvulsants drugs in several combinations, piridoxin, piridoxal-phosphate and vitamin cofactors were tested without success. At two months of life, he presented cluster of epileptic spasms preceded by focal seizures, associated with hypsarrhytmia in the EEG recording. The ketogenic diet reversed the hypsarrhythmia and spasms, but focal migrating seizures didn’t disappear (Figure 1). His examination, at three months of life, showed central hypotonia, decrease of general movements and arrest of the sensorial functions. Whole Exome Sequencing (WES) revealed a heterozygous, de novo, missense mutation in KCNT1 (c. 1429G>A; p. Ala477Thr). When the patient was seven months old, he started with quinidine treatment, after his parents consented to its off-label use. At that time, he had more than 20 seizures per day, he followed a combination of valproate, levetiracetam and clonazepam treatment. Progressive increasing of quinidine dose was performed, with blood levels monitoring and electrocardiogram studies.
Figure 1:
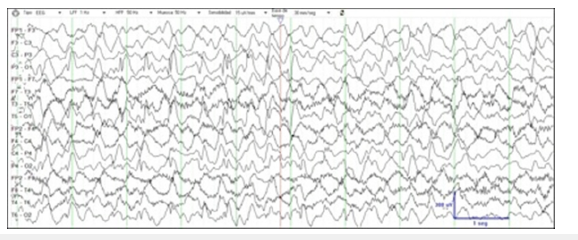
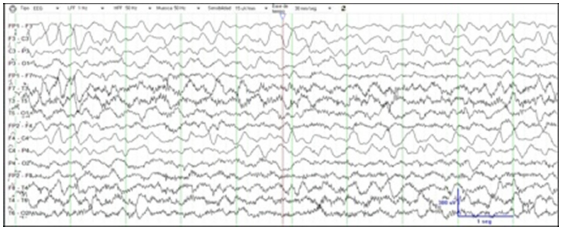
After four weeks on his target dose of quinidine (30mg/Kg/ day) seizures were decreased to 5-10 per day. After two months he had 2-4 seizures per day and showed an improvement in his social and motor development. Later, quinidine dose was adjusted according to plasma levels (2-5mcg/ml), and seizures became more frequent during intercurrent illness or dental eruption. Between 1 and 3 years old, when seizures exacerbated, a dose escalation produced a significant improvement in the following weeks. At this time, seizures have decreased to 1-3 per week. Muscular tone improvement, with stable sitting position and beginning of standing, better visual attention and babbling are noticed in last clinical assessment at three years old. Video-EEG recording showed a marked improvement (Figure 2). Prolongation of the QT interval has not been observed so far.
Figure 2:
Discussion
EIMFS is a devastating condition to lead to an encephalopathic syndrome in a large majority of cases. Seizure control, particularly in early phases of the syndrome, may improve outcomes. Since genetic basis has implications for treatment, screening a panel of genes associated with EIMFS may be the most efficient strategy. Disease-specific treatments are available for only a minority of severe epilepsies with genetic causes, such as the ketogenic diet in patients with glucose transporter 1 deficiency due to SLC2A1 mutations [10]. The aim of targeted therapies is not only to improve seizure control, but also to prevent developmental delay or encephalopathic evolution.
Quinidine, that causes a reversible block in KCNT1 channels, was able to reverse mutation-specific gain-of-function properties in vitro [11]. So far, ten paediatric patients with KCNT1 mutations to which quinidine treatment was given, have been reported in the literature [8,11-13]. Response was observed in some, but not in others [12,13]. In six children under three years old the response was positive. Mullen et al. [13] performed a double-blind placebocontrolled trial of oral quinidine for seizures in 6 adults’ patients with ADNFLE due to KCNT1 mutations. They concluded that quinidine did not show efficacy in adults with ADNFLE. Quinidine might be a rational therapy for seizure control in cases of EIMFS associated with KCNT1 mutation [11]. However, caution must be implemented when using quinidine, since could cause a long QT interval and lead to cardiac arrest. Besides, KCNT1 is also expressed in cardiomyocytes and KCNT1 mutations may be associated with cardiac arrhythmias and Sudden Unexpected Death in Epilepsy (SUDEP) [6]. The patient underwent Electrocardiographic (ECG) monitoring when quinidine dosages were increased, without appreciate QT interval prolongation.
In conclusion, the effects of quinidine in EIMFS due to KCNT1 gain-of-function mutation remain unknown. The poor CNS penetration makes difficult to determine which dose is the most appropriate, and gradual increase of dosing over time is limited by its effects on the heart. According to another Madaan et al. [13] we think that the onset of quinidine treatment in early stages of development helps to improve seizures control and neurodevelopmental disabilities. Besides, since some patients who were treated with quinidine only for one or two months [12,13] hadn’t positive response, it may be possible an additive response along the time to quinidine.
References
- Ohba C, Kato M, Takahashi N, Osaka H, Shiihara T, et al. (2015) De novo KCNT1 mutations in early-onset epileptic encephalopathy. Epilepsia 56(9): e121-128.
- Kearney JA (2016) Locus heterogeneity in epilepsy of infancy with migrating focal seizures. Epilepsy Curr 16(1): 43-45.
- Barcia G, Fleming MR, Deligniere A, Gazula VR, Brown MR, et al. (2012) De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet 44(11): 1255-1259.
- Møller RS, Heron SE, Larsen LH, Lim CX, Ricos MG, et al. (2015) Mutations in KCNT1 cause a spectrum of focal epilepsies. Epilepsia 56(9): e114-120.
- Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, et al. (2003) The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 37(5): 765-773.
- Lim CX, Ricos MG, Dibbens LM, Heron SE (2016) KCNT1 mutations in seizure disorders: the phenotypic spectrum and functional effects. J Med Genet 53(4): 217-225.
- Kaczmarek LK (2006) Non-conducting functions of voltage-gated ion channels. Nat Rev Neurosci 7(10): 761-771.
- Dilena R, DiFrancesco JC, Soldovieri MC, Giacobbe A, Ambrosino P, et al. (2018) Early treatment with quinidine in 2 patients with Epilepsy of Infancy with Migrating Focal Seizures (EIMFS) due to gain-of-function KCNT1 mutations: functional Studies, clinical responses, and critical issues for personalized therapy. Neurotherapeutics 15(4): 1112-1126.
- McTague A, Howell KB, Cross JH, Kurian MA, Scheffer IE (2016) The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol 15(3): 304-316.
- Bearden D, Strong A, Ehnot J, DiGiovine M, Dlugos D, et al. (2014) Targeted treatment of migrating partial seizures of infancy with quinidine. Ann Neurol 76(3): 457-461.
- Abdelnour E, Gallentine W, McDonald M, Sachdev M, Jiang YH, et al. (2018) Does age affect response to quinidine in patients with KCNT1 mutations? Report of three new cases and review of the literature. Seizure 55:1-3.
- Madaan P, Jauhari P, Gupta A (2018) A quinidine non responsive novel KCNT1 mutation in an Indian infant with epilepsy of infancy with migrating focal seizures. Brain Dev 40(3): 229-232.
- Mullen SA, Carney PW, Roten A, Ching M, Lightfoot PA, et al. (2018) Precision therapy for epilepsy due to KCNT1 mutations: A randomized trial of oral quinidine. Neurology 90(1): e67-e72.
© 2024 Lütfiye Kaya Cicerali. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






