- Submissions

Full Text
Research in Pediatrics & Neonatology
Monitoring of Regional Cerebral Oxygenation after Red Blood Cell Transfusion in the Preterm Infant
Silveira V, Tejeira S, Vaamonde L, Rodríguez M and Blasina F*
Department of Neonatology, University Hospital, Universidad de !a Republica, Uruguay
*Corresponding author: Fernanda Blasina, Department of Neonatology, University Hospital, Universidad de la Republica, Uruguay, Tel: 59824871515; Email: fblasina@gmail.com
Submission: October 13, 2017; Published: January 18, 2018

ISSN : 2576-9200Volume1 Issue4
Introduction
In Uruguay, there are about 47,000 births per year, of which approximately 10% are born before 37 weeks of gestational age [1]. Anemia is one of the most frequent complications of prematurity and it compromises the neurological development of the newborn. The factors responsible for anemia of prematurity, and its high RBC transfusion rate are linked to iatrogenic, physiological, and hematopoietic immaturity system factors. It has been stated that the amounts of blood withdrawn from preterm for laboratory testing are the main cause of anemia in this group of patients. All newborns experience a decline in hemoglobin (Hb) concentration during the first week of life.
This decline results from multiple physiological factors as well as additional factors in preterm neonates. Premature infants do not complete the third trimester of gestation and it is at this stage that occurs the highest iron transport through the placenta. On the other hand, at this stage of development erythropoiesis mainly occurs in the liver and in the bone marrow. The liver is less sensitive to anemia in the generation of an erythropoietin response; thus, it will be less intense. All mechanisms such as: rapid growth, multiple blood extractions and inadequate erythropoiesis contribute to anemia and its severity [2].
Moreover, symptoms of anemia are related to deficiency of tissue oxygenation because of a decrease in the oxygen supply. One of the most frequently used treatments for the correction of this deficiency is the RBC transfusion. Despite exposure to various risks related to this treatment, during hospitalization, 80% of preterm infants require RBC transfusion [3]. In addition, the indication of transfusion remains controversial [4], and its adverse effects are still under discussion [5]. However, the Hb and/or hematocrit (Hct) thresholds that determine a decrease in oxygen supply and demand by the tissues, mainly at brain level have not been systematically evaluated.
This is because the metabolic demands of oxygen vary in the different clinical situations, as well as in the different stages of postnatal development, and among individuals. Studies comparing restrictive versus liberal RBC transfusion which have been implemented have not yet demonstrated superiority of one type of criterion against the other, especially in neurological development [6-8]. Moreover, it has been shown that having an institutional policy for RBC transfusion and having a methodology to implement it reduces the number of RBC transfusions [9,10]. Therefore, the effort to find elements that collaborate in the development of an institutional policy plays a key role in the optimization of the use of RBC transfusion, which is a critical concept for guiding our work.
The use of NIRS provides substantial data on the kinetics of oxygen at the brain and it reflects the physiological variability of each newborn. The NIRS allows us the assessment of the percentage of oxygenated Hb in the brain in relation to the total Hb, of which 70% corresponds to the venous bed, by measuring the regional brain or somatic O2 saturation (rcSO2 and rsSO2 respectively). This non-invasive monitoring technology allows us to better understand cerebral oxygenation by means of calculating FOE which represents the balance between the amount of O2 delivery and extraction. FOE gives us more information about cerebral O2 metabolism. It also indirectly allows us the assessment of cerebral perfusion because rcSO2 depends on the blood flow of the monitored region.
NIRS is a technology that helps determining if in the course of anemia there is a decrease in the O2 delivery to the tissues, mainly to the brain, since it is one of the most vulnerable and sensitive organ in the face of increases and decreases of O2 concentration [5]. Thus, this work aimed to study the variation of regional brain oxygenation in preterms that required RBC transfusion.
Materials and Methods
This work was carried out after the approval of the Bioethics Committee of Hospital de Clinicas of the Facultad de Medicina, Universidad de la Republica. A retrospective descriptive study was performed. This study enrolled patients admitted to the intensive care unit of the Neonatology Department of the Hospital de Clinicas from January 1st, 2014 to October 31st, 2016. The inclusion criteria were: newborns with anemia of prematurity requiring RBC transfusion with a gestational age, at the time of RBC transfusion, between 27 and 35 completed weeks. The indication of RBC transfusion was according to the following criteria: Ventilated patients with FiO2 requirement >0.4 and an Hct≤30%; Patients ventilated with a FiO2 requirement <0.4 and an Hct ≤25%; Patients with supplemental O2 requirement, who have an Hct ≤20% and tachycardia for more than 24 hours or decrease in the growth rate or increased O2 requirements in the last 48 hours [11].
Exclusion criteria for this study were: all patients older than 35 weeks of gestational age, presence of malformations in the central nervous system, previous diagnosis of hemoglobinopathies and patients undergoing a generalized infection. The variables analyzed were rcSO2, pulse saturation by pulse oximetry (SatO2), anthropometric data of weight, height, days of life, type of ventilation-in case of mechanical ventilation or noninvasive ventilation- and clinical symptoms of anemia.
Data collection
Data collection was performed by analyzing the medical records of patients enrolled in this study. Patients who met the inclusion criteria were monitored by NIRS (INVOSTM 5100) for a period of 12 hours before the beginning of transfusion up to 12 hours after ending it, as shown in (Figure 1A). With the patient in the supine position a NIRS sensor was placed on the skin of the frontoparietal side of the neonatal skull, adjusting its position according to the characteristics of the cephalic convexity of each patient, in order to obtain the best signal/noise ratio in the record. With regard to the region where the sensor was positioned, several studies showed that there are no differences in rcSO2 values between the frontal and temporol-occipital regions [12].
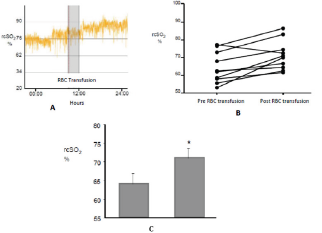
Figure 1: Increase of the rcSO2 after the RBC transfusion.
A shows the recording of a patient during 24h including the moment of transfusion (in grey).
B Variation of the recording for each case.
C shows the media of the increase of rcSO2 before and after RBC transfusion. P<0. 05.
Since the patients were preterm, including some very immature whose skin is fragile, the self-adhesive sector of each sensor was protected with a hydrocolloid compound based on carboxymethylcellulose sodium, pectin and gelatin, to avoid the direct fixation of the sensor to the skin. In this way the sensor was placed on each region with an elastic headband avoiding skin lesions. NIRS enable real time measurement of the rcSO2 by emitting near infrared light from sensors with LED lights. Each set of sensors has two detectors of different depth range from the LED emitter, allowing the measurement of two depths. The records obtained by this technology are stored in digital format in the database of the unit.
The data of the transfused patients obtained by the NIRS began to register 12hours before the transfusion (t0) and then every 3 hs: t1, t2, t3, etc. The total duration of the registry varied depending on the clinical situation of each patient.
Data analysis
The NIRS recorders were analyzed with the COVIDIEN INVOS Analytics Tool - Version 1.2. program, as well as transferred to a computer and analyzed with OpenOffice, Text Document and Spreadsheet programs.
FOE was calculated using the following equation:
FOE =VO2/DO2
VO2 (Consumption of O2) = cardiac output x (Hbx1.39) x (sat aO2-sat vO2)
DO2 (Delivery of O2) = cardiac output x (Hb x 1.39) x sat aO2.
Then we can simplify the equation as follows:
FOE = peripheral SatO2 - rcSO2
Peripheral SatO2
Statistical analysis
Descriptive statistics were computed for all variables. Data was summarized as mean ± SD and Student's Test was performed to determine the statistical significance. It was considered statistically significant p<0.05. Statistical correlation between different variables was performed.
Results
The study included 10 RBC transfusions in 5 patients. The patients' baseline characteristics are given in (Table 1). The mean of the gestational age at birth was 27 weeks (±2), and birth weight was 985g (±182g); the Apgar test at the first minute was 3 to 9 and at 5 minutes from 6 to 10. For this group of patients the Hb at birth was 15.5g/dl (±1.4) and the Hct was 37.6% (±6.7). Patients' characteristics at the time of the RBC transfusion are shown in Table 2.
Table 1: Patient Characteristics.
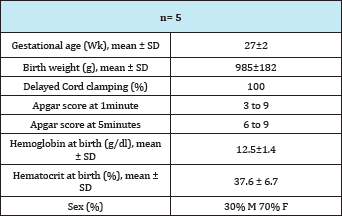
Table 2: Characteristics of patients at the time of transfusion.
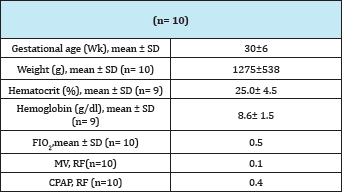
Patients were transfused at 31 (±6) weeks of gestational age and their weight was 1275g (±538). The value of Hb at the time of RBC transfusion was 8.6 g/dl (±1.6) and Hct 25.0% (±4.5). Half the patients who participated in this study were receiving oxygen therapy at the time of RBC transfusion. Patients seldom required mechanical ventilation (MV). At the time of RBC transfusion only one patient required MV and 4 patients were receiving continuous positive airway pressure (CPAP). Regarding rcSO2 records it can be said that no patient presented values lower than 55%; that is, all patients presented rcSO2 values within the range considered the lowest limit of normality [13-15]. (Figure 1A) shows the recording of a patient during the RBC transfusion where it can be observed the increase of the rcSO2 after the RBC transfusion. (Figure 1B) shows the variation of the rcSO2 for each of the cases analyzed. A baseline value of 64% (±2) and post-transfusion of 71% (±2) was obtained for the total of the RBC transfusions, these variation was statistically significant (p<0.05) as it is represented in the (Figure 1C).
Figure 2: Variation of the FOE. A shows the recording of a patient during time. The arrow represents the RBC transfusion. B shows the media of FOE pre and post RBC transfusion for the whole group. P<0. 05.
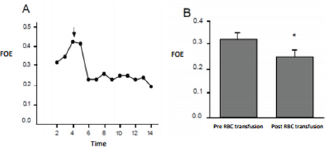
We observed a significant decrease in the cerebral FOE after the RBC transfusion. (Figure 2A) shows the variation of the cerebral FOE for one of the patients included in our study. This variation after the RBC transfusion was observed for the whole group as it is shown in (Figure 2B). The cerebral FOE decrease from 0.32 to 0.25 after the RBC transfusion, this result is statistically significant, which means that prior to the RBC transfusion the overall brain extraction approached 32%, falling to about 25%.
Discussion
NIRS technology has been used for several years in neonatal units. Continuous real time noninvasive monitoring can provide relevant information about the tissue oxygenation in function of the circulating O2 [16]. In Uruguay, the first equipment available in intensive care, whether neonatal, pediatric, or adult, entered the Department of Neonatology, University Hospital, Universidad de la Republica, Montevideo in March 2013, as a donation by a former Professor of the Department, currently Professor Emeritus of the Faculty of Medicine, Universidad de la Republica, Montevideo, Dr. Martell. From that moment, the Department of Neonatology has had the opportunity to make the learning curve for the proper use of this equipment. One of the main topics that motivated the learning of this new technology was its usefulness in a very frequent pathology such as anemia of prematurity, making its utility indisputable in such situation.
In the whole group of patients, the NIRS has proved to be a non- invasive, useful and reproducible tool with the potential to provide elements that help to indicate a RBC transfusion and it is an excellent tool for monitoring the efficacy of this treatment. Continuous monitoring using NIRS did not interfere with patient care. As for the costs of using this equipment, the fungibles are focused on the consumption of sensors that are costly (approximately US$ 140 each), not reusable, which is a limitation in patient monitoring. This study confirms statistically significant increase in the rcSO2 after the RBC transfusion. This result agrees with others obtained in other neonatal units.
The increase in post-transfusion rcSO2 is due to an increase in the oxygenated Hb level [15]. Such result is fundamental since it shows that the use of NIRS monitoring allows us the evaluation of the impact of the treatment of anemia by the RBC transfusion in the region studyed, allowing to control the quality of the procedure and the expected response to the treatment. This fact shows that the NIRS constitutes an adequate tool to assess the efficacy of the RBC transfusion in the preterm newborn.
The cerebral FOE showed a statistically significant decrease 12 hours after the RBC transfusion. The decrease of the cerebral FOE can be transient after the RBC transfusion and it could be due to the abrupt increase of the concentration of oxygenated Hb. The increase in the availability of O2 at the cerebral level, with constant O2 consumption indicates a decrease in the FOE [17]. Some authors have stated that there is a negative correlation between FOE and Hb concentration, as well as an increase in FOE versus a decrease in PaCO2 [18]. These authors have attributed the decrease in FOE, after transfusion, to a transient decrease in cerebral blood flow, in response to the increase in Hb concentration. In this work, the variations of the cerebral blood flow were not directly or indirectly explored, so it is not possible to relate the variation of the FOE to this regional hemodynamic parameter.
The usefulness of having NIRS for monitoring a preterm patient with anemia has been noted in some studies, even suggesting its incorporation as one of the decision criteria for a RBC transfusion in preterms. Recently, it has been shown that infants with initial rcSO2 below 55% have a significant decrease of the apneas, after the transfusion, compared to those patients who have a level of rcSO2 over 55% [14] prior to treatment.
The NIRS is used for the monitoring of different brain and somatic regions of patients, as has been pointed out [13,19] it has increased the number of utilities in different pathologies. A fundamental role of NIRS in the monitoring of the premature newborn is to avoid episodes of hypoxia and hyperoxia since it constitutes a complementary and very sensitive system of noninvasive monitoring of cerebral oxygenation. In these preterm patients avoiding these episodes is relevant since it has been stated that the damage that they produce at brain level has implications in neurodevelopment.
On the other hand, the NIRS has been used for the monitoring of rcSO2 in premature patients with patent ductus arteriosus with hemodynamic repercussion. In this group of patients it has played a key role in assessing treatment and preventing complications [20]. NIRS has been suggested as a noninvasive tool for detecting low splanchnic perfusion and therapeutic evaluation; its relation to different feeding techniques (bolus or continuous feeding) is currently subject of research [21]. These changes in regional saturation are presumed to be a potential splanchnic perfusion biomarker in preterm infants, useful in limiting local damage and avoiding morbidities.
This work has allowed us to know the basics of using NIRS technology as a useful tool for the monitoring of critical patients, reflecting situations of both pathophysiological stability and dynamic phenomena that need to be further studied, representing a kick for future research. On the other hand, we believe that our results provide evidence about the role of this new technology in the neonatal units, especially in Latin America.
References
- Ministerio de Salud Publica (2009) Division de Epidemiologi'a. Uruguay, South America.
- Strauss RG (2010) Anemia of Prematurity: Pathophysiology and treatment. Blood Rev 24(6): 221-225.
- Bifano EM, Curran TR (1995) Minimizing donor blood exposure in the neonatal intensive care unit. Current trends and future prospects. Clin Perinatol 22(3): 657-669.
- Venkatesh V, Khan R, Curley A, Hopewell S, Doree C, et al. (2012) The safety and efficacy of red cell transfusions in neonates: a systematic review of randomized controlled trials. Br J Haematol 158(3): 370-385.
- Andersen C C, Keir AK, Kirpalani HM, Stark MJ (2015) Anemia in the Premature Infant and Red Blood Cell Transfusion: New Approaches to an Age-Old Problem. Current Treatment Options in Pediatrics 1(3): 191201.
- Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, et al. (2005) Randomized Trial of Liberal Versus Restrictive Guidelines for Red Blood Cell Transfusion in Preterm Infants. Pediatrics 115(6): 1685-1691.
- Kirpalani H, Whyte R K, Andersen Ch, Asztalos E V, Heddle N, et al. (2006) The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr (149): 301-307.
- Chen HL, Tseng HI, Lu CC, Yang SN, Fan HC, et al. (2009) Effect of blood transfusions on the outcome of very low body weight preterm infants under two different transfusion criteria. Pediatr Neonatol 50(3): 110116.
- New HV, Berryman J, Bolton Maggs PH, Cantwell C, Chalmers E A, et al. (2016) Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol 175(5): 784-828.
- Baer VL, Henry E, Lambert DK, Stoddard R A, Wiedmeier S E, et al. (2011) Implementing a program to improve compliance with neonatal intensive care unit transfusion guidelines was accompanied by a reduction in transfusion rate: a pre-post analysis within a multihospital health care system. Transfusion 51 (2): 264-269.
- Robin K Ohls MD Why, When, and How Should We Provide Red Cell Transfusions and Erythropoiesis Stimulating Agents to Support Red Cell Mass in Neonates? Hematology, Immunology and Infectious Disease: Neonatology Questions and Controversies, pp. 5774.
- Wijbenga RG, Lemmers PM, van Bel (2011) Cerebral oxygenation during the first days of life in preterm and term neonates: differences between different brain regions. Pediatric Res 70(4): 389-394.
- Wolf M, Greisen G (2009) Advances in near- infrared spectroscopy to study the brain of the preterm and term neonate. Clin Perinatol 36(4): 807-834.
- Seidel D, Blaser A, Gebauer C, Pulzer F, Thome U, et al. (2013) Changes in regional tissue oxygenation saturation and desaturation after red blood cell transfusion in preterm infants. J Perinatol 33(4): 282-287.
- Alderliesten T, Dix L, Baerts W, Caicedo A, Van Huffel S, et al. (2015) Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res 79(1-1): 55-64.
- Charles Keinman, Istvan Seri, Serie Richard A, Polin (2011) Espectroscopi'a en el infrarrojo cercano y su utilization para la evaluation de la perfusion tisular en el recien nacido. Suresh Victor and Michael Weindling Cardiologi'a y hemodinamica.
- Naulaers G, Meyns B, Miserez M, Leunens V, Van Huffel S, et al. (2007)Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology 92(2): 120-126.
- Wardle SP, Yoxall CW, Weindling AM (2000) Determinants of cerebral fractional oxygen extraction using near infrared spectroscopy in preterm neonates. J Cereb Blood Flow Metab 20(2): 272-279.
- Sood BG, Mc Laughlin K, Cortez J (2015) Near-infrared spectroscopy: applications in neonates. Semin Fetal Neonatal Med 20(3): 164-172.
- Wolf M, Naulaers G, Van Belc F, Kleiser S, Greisen G (2012) A Review of near Infrared Spectroscopy for Term and Preterm Newborns. Journal of Near Infrared Spectroscopy 20(1): 43-55.
- Corvaglia L, Martini S, Battistini B, Rucci P, Aceti A, et al. (2014) Bolus vs. continuous feeding: effects on splanchnic and cerebral tissue oxygenation in healthy preterm infants. Pediatric research 76(1): 81-85.
© 2018 Silveira V, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






