- Submissions

Full Text
Research & Development in Material Science
Elucidating Chemo-Mechanical Synthesis and Microstructural Study on the Performance of Partial Cement-Based Concrete Composites Against Sulphate Attack – A Review
Hafiz Muhammad Nadir1 and Ash Ahmed2*
1PhD Researcher, Civil Engineering Group, School of Built Environment & Engineering, Leeds Beckett University, UK
2Associate Professor, Civil Engineering Group, School of Built Environment & Engineering, Leeds Beckett University, UK
*Corresponding author: Ash Ahmed, Associate Professor, Civil Engineering Group, School of Built Environment & Engineering, Leeds Beckett University, Civic Quarter Northern Terrace Leeds, LS2 8AG, UK
Submission: December 12, 2022;Published: December 22, 2022

ISSN: 2576-8840 Volume 18 Issue 2
Abstract
The well-known weakness of cement concrete against external/internal sulphate attack and an estimated 7-10% global greenhouse gas emission by the construction industry (mainly contributed by cement manufacturing and supply have encouraged researchers to elucidate the chemical synthesis taking place in the preparation and hydration of cement concrete along with the factors affecting the sustainability of hardened concrete. In this review study, an endeavour has been made to explore the use of Supplementary Cementitious Materials (SCMs) of different hydrocarbon compositions, including organic/ inorganic compounds like pozzolans derived from natural (zeolite/ metakaolin derived from kaolinite), agricultural (rice husk ash, corn cob ash) and industrial fields Pulverised Fly Ash (PFA), Silica Fume (SF) and a renowned cement replacement material, i.e., Ground Granulated Blast Furnace Slag (GGBS),). The partial replacement of 0-30% pozzolans with cement as a binder has been reviewed objectively to achieve economic/ environmental benefits by enhancing strength and durability against dangerous sulphate attacks. The chemo-mechanical synthesis involving SCMs has been explored to understand the formation of additional calcium silicate hydrate C-S-H gel by blending various pozzolans. The research elucidates an improvement in strength up to optimum ratios of 1-15% for different SCMs. However, the strength was observed to reduce beyond a certain % ratio of SCMs blending due to the formation of expansive alkaline silica hydroxide gel, which causes cracking and weak structure. The aviation industry is considered the top emitter of CO2 (3% of total global emissions), however, the construction industry emits 7-10% of global greenhouse gases, which is nearly three times greater. Therefore, the supportive use of up to 90% SCMs can result in a significant reduction of CO2 by the construction industry based on the type/ratio of blending SCMs. Microstructural studies using scanning electron microscopy SEM and X-ray Diffraction (XRD) have also been explored. These microstructural studies have further clarified the development of ettringite in concrete after sulphate attack and the beneficial use of pozzolans to a certain extent to prevent the formation/ propagation of ettringite-specific cracks in the micro/ nano-pores of concrete structures. In general, research has shown that the addition of SCMs in concrete results in an increase in strength and superior resistance to sulphate attack.
Keywords: Chemical synthesis; Sulphate attack; Pozzolans-based SCMs; Mechanical properties; Microstructural scanning
Introduction
Technological advancement has enabled engineers to produce masterpieces of construction in diverse geographical/ ecological locations by harnessing nature’s power, enabling a comfortable human life but subjecting the environment and infrastructure to different chemical / pollution hazards [1]. The use of lime and natural volcanic pozzolans has been in use since ancient civilisations [2]. The invention of ordinary Portland cement in the 1860s suppresses the use of lime/ pozzolans due to its swift setting time and easy insitu handling [2,3]. Still, it resulted in up to 10% of global CO2 emissions [4]. The cement concrete was found to be highly vulnerable to the ingress of sulphates and chlorides in marine environments [2-8]. Therefore, the researchers focussed on the formulation of greener alkali activators [9-13] as supplementary cementitious materials SCMs [14] to address cement concrete vulnerabilities [15,16]. The research suggests that using pozzolans in cement concrete as SCMs to specific ratios improves mechanical properties and prevents the ingress of moisture-containing harmful minerals/ chemicals by reducing porosity, permeability and creating defence layers against sulphate internal/ external attacks [8,17-20]. In this paper, a review study has been conducted to explore the beneficial performance of SCMs-based concrete composites under accelerated sulphate attacks.
Review of chemical synthesis in cement hydration and sulphate attack
The chemistry of cement hydration:R.H. Bogue identified the main four ingredients of cement hydration in 1960, which include alite (C3S tricalcium silicate 3CaO.SiO2), belite (C2A dicalcium silicate 2CaO.SiO2), celite (C3A tricalcium aluminate 3CaO.Al2O3) and felite (C4AF tetracalcium alumina ferrite 4CaO.Al2O3.Fe2O3) [10,21- 24]. Alite starts the cement hydration process and is considered the major initial strength-imparting compound, followed by belite, which is considered to impart the latter strength of concrete by the formation of calcium silicate hydrate (3CaO.2SiO2.3H2O), also known as C-S-H gel (equations 1,2) [10,25,26].

Equation 1
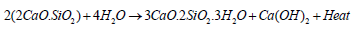
Equation 2
The third compound, “celite”, contributes to the flash-setting property of cement and does not impart any significant strength to concrete. To prevent flash setting of cement, gypsum (CaSO4) is added to cement, but it reacts with celite and produces hazardous long needle-like crystals of 3CaO.Al2O3.CaSO4.32H2O, called ettringite which, is responsible for internal sulphate attack and cracking of hardened cement/ concrete paste, as shown in equation 3 [10,27-33].

Equation 3
However, when gypsum is fully depleted during the reaction, then celite starts to hydrate internally formed ettringite to convert it to a 2.5 times lighter sulphate deficient compound of monosulphate aluminate hydrate (3CaO.Al2O3. 3CaSO4.12H2O) which cover the cement paste to stop flash setting and reformation of ettringite, thus making concrete a durable material internally as shown in equation 4 [10,29,33,34]. Due to monosulphate aluminate hydrate, the concrete will remain stable in a sulphate-deficient environment. Still, it will reconvert to ettringite-formation when exposed to sulphate-abundance in the form of ingress of sulphateladen moisture from external sources, also known as external sulphate attack [10,34].

Equation 4
The fourth compound of cement felite or ferrite is mainly used as a filler material to decrease porosity in the hardened concrete by the formation of ferric aluminate hydrates, which is finally converted to a filler material called garnets (monosulphate ferric aluminium hydrates) as shown in equations 5 and 6 [25,29,33]. The final cement paste contains around 60% C-S-H gel, an estimated 20% ettringite, 15% Ca(OH)2 and 5% voids/ entrapped air [10,29].

Equation 5
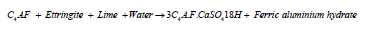
Equation6
The synthesis of external/ internal sulphate attacks:The formation of monosulphate aluminate hydrates prevents internal sulphate attacks and stops further crack propagation/ instability inside hardened concrete [10,35]. However, when hardened concrete is exposed to solutions containing sodium or magnesium sulphate (Na2SO4/ MgSO4) through ingress from external resources [36], this monosulphate aluminium hydrate absorbs SO-4. It releases Ca++ in the exchange of cations/ anions to reconvert to long needles of ettringite and CaSO4 (gypsum), brucite Mg(OH)2 or NaOH based on the type of sulphate solutions [10,35,37]. The Na2SO4 results in expansion, and MgSO4 reduces the strength of the concrete structure [10,37-39]. The cations Mg++/ Na+ and Ca++ exchange with anions SO-4 and OH- exchange. The sulphate ion SO-4 frommagnesium/ sodium sulphate transfers inwards to form gypsum CaSO4, whereas the OH- ion from Ca(OH)2 exchanges outward to form brucite Mg(OH)2 or NaOH (equation 7,8) [10,40-45].
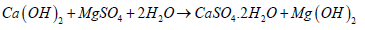
Equation 7
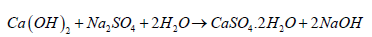
Equation 8
The Mg++ exchanges with Ca++ of C-S-H gel and forms magnesium silicate hydrate gel which has no strength and converts the concrete into a ‘mushy’ material giving a big blow to lose its strength/ hardness (equation 9) [10,46].

Equation 9
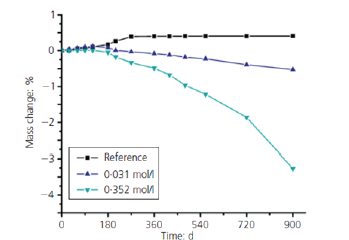
The researchers conducted a microstructural and qualitative analysis of OPC paste cubes of 20mmx20mm by placing them in water (reference readings) 0.03 mole/l (0.5% by weight) and 0.35 mole/l (5% by weight) Na2SO4 solution for up to 900 days. The cubes were examined for the assessment of surface deterioration and crack propagation on 90,180, 300, 600 and 900 days. The lightly concentrated 0.5% Na2SO4 inflicted slight cracking on edges after 180 days, and slight spalling of cubes was observed after 900 days. However, the cubes placed in 5% Na2SO4 solution started considerable cracking after 90 days on the edges, which were found to spread to the surface of the cubes after 180 days. The ingress of Na2SO4 solution into the inner body of cubes started after 300 days, and considerable peeling and spalling were observed in the cubes due to the formation of gypsum in the veins. After 600 days, significant spalling and peeling of the outer layer was observed due to expansion caused by the development of long ettringite needle crystals inside the outer and inner cores of cubes and the decalcification of C-S-H gel. After 900 days complete outer layer was found to be peeling, cubes started bulging due to expansion/ spalling and loss in mass was observed, showing a significant impact of sulphate attack on cement paste cubes as shown in Figure 1; [41]. A reduction of 64% compressive strength (from 70MPa to 25MPa) and 3% mass loss were observed in 5% Na2SO4 after 900 days, as shown in Figures 2 & 3; [41].
Figure 1:Qualitative Analysis of OPC cubes in 0.5% and 5% Na2SO4 solution after 900 days [41].
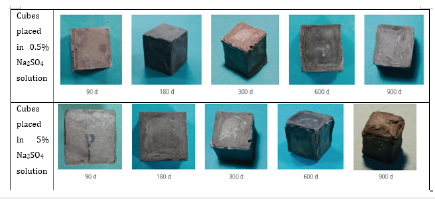
Figure 2:Reduction in compressive strength of OPC cubes in 0.5% and 5% Na2SO4 solution after 900 days [41].

Figure 3:Reduction in the mass of OPC cubes in 0.5% and 5% Na2SO4 solution after 900 days [41].
A microstructural analysis was conducted on these cubes to assess the ingress of sulphate solution, propagation of cracks, development of gypsum and ettringite crystals in the cubes and peeling off surface/ loss of mass due to sulphate attack after 900 days. Scanning electron microscopy (SEM) was done after 600 and 900 days on the surface (Figure 4a) and 1mm under the surface (Figure 4b). The sulphate attack has been described as impacting the cubes in four stages. In the first stage, sulphate ions penetrate the surface, react with Ca++ and OH- ions, and form monosulphate. In the second stage, decalcification of C-S-H gel starts and CaSO4 is produced in the veins/cracks. In the third stage, cracks propagate, and gypsum is depleted by converting C-S-H gel into ettringite. In the fourth stage, the sulphate ions keep consuming the Ca++ from C-S-H gel and convert it into mushy Na-S-H gel having no strength and ultimately resulting in spalling, peeling, loss of mass and reduction in strength. The concentration of sulphate solution, permeability, period of exposure and cement composition influence the degree of sulphate attack/deterioration [10,41].
Figure 4:Microstructural Analysis of OPC cubes by SEM in 5% Na2SO4 solution after 600 and 900 days [41]..

Use of pozzolans as partial SCMs in cement concrete for prevention of sulphate attack:
The researchers have been experimentally devising different composites containing cement, lime and waste materials/derivatives from industrial/agricultural and natural resources having an abundance of metals oxides like silica, alumina, ferric oxide etc., making them a good pozzolan at par containing a total of 60% or more pozzolanic material. These materials have got an exhaustive list and generally include pozzolans/cement replacement materials like Ground Granulated Blast Furnace Slag (GGBS), Pulverised Fly Ash (PFA), Silica Fume (SF), Metakaolin (MK), Rice Husk Ash (RHA), Palm Ash (PA), Corn Cob Ash (CCA) and zeolite etc. The silica in these materials reacts with Ca(OH)2 to produce an increased quantity of C-S-H gel, as shown in equation 10 [10,48]. However, an increased quantity of pozzolans results in excess production of alkaline silica hydroxide (Si(OH)4), which remains in pores as an aqueous solution Si(OH)4. It has got swelling properties which produce cracks and result in instead weakening of concrete [10,47-72].

Results and Discussion of Different Case Studies/ Microstructural Analysis
Performance of established pozzolans/ cement replacements like GGBS, PFA, SF and MK as binary and ternary SCMs with OPC against sulphate attack
Contemporary research had shown an improvement in the sustainability of concrete when ternary concrete mixes were used in different sulphate solutions compared to binary cement concrete with different pozzolans [10,50,60]. The use of GGBS, PFA, SF and MK in cement concrete is already in practice due to its enhanced environmental benefits. Their use in cement concrete as a partial replacement being a good SCM is an established fact in the construction industry [10,62,65,66].
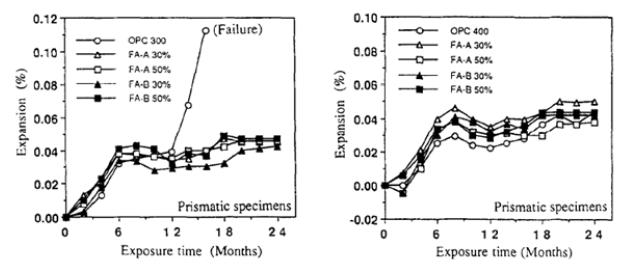
A detailed experimental study was conducted on the improved sulphate resistance of GGBS blended cement concrete. He blended 60% and 70% GGBS with 40% and 30% OPC along with 2% and 3% CaSO4 and 3% CaCO3 and immersed cubes and prisms in Na2SO4 and MgSO4 solutions for six years to observe expansion and reduction in compressive strength. The tests were conducted after 3,7,28 days, and 1,2 and 6 years. The study revealed that 60% GGBS-cement composite with 3% lime and 3% CaSO4 performed the best in all the testing regarding compressive strength and expansion parameters, as shown in Table 1; [70]. The consistent efficacy of GGBS-based cement composite exhibited beneficial impacts against external sulphate attack of concentrated Na2SO4 and MgSO4. The presence of lime and CaSO4 provided the Ca++ and SO3- - cations and anions in a chemical reaction which prevented annihilation/ de-calcination of C-S-H gel during the formation of ettringite and gypsum on external sulphate attack by concentrated sulphate solutions. The study shows a beneficial use of GGBS as SCM with lime and CaSO4 for the long-term durability of concrete [70]. OPC cubes were almost wholly disintegrated after five years; 60% of GGBS cubes also impacted more in Na2SO4 than in MgSO4. 70% GGBS composite performed the best in both solutions, especially with higher percentages of lime and CaSO4, elucidating that the increased quantity of GGBS performs better in a sulphate environment [70]. The prisms were tested for durability (expansion) in Na2SO4 and MgSO4 solutions. The prisms were prepared using composites of OPC with 60% and 70% GGBS with the addition of 2% and 3% CaSO4 and 3% CaCO3. Prisms were immersed in Na2SO4 solution for 1 and 3 years, whereas the duration of immersion was extended to 6 years in MgSO4 solution. The promising results exhibited an increased quantity of GGBS up to 70% with higher quantities of CaSO4 and CaCO3 performed better by showing negligible expansion/ surface erosions, followed by 60% GGBS composites as the second-best performer in the sulphate environment. At the same time, control mix prisms comprising only OPC performed the worst by showing considerable surface wear and tear with up to 0.1% expansion in the first nine months of emersion in sulphate solutions. The results reassured that GGBS composites perform better in sulphate attacks by lesser production of ettringite and increased output of C-S-H gel during the hydration process due to pozzolanic reactions, as depicted in Table 1; [10,70].
Table 1:Compressive strength of GGBS composites in water and sulphate solutions [70].

Figure 5:Compressive strength of low/high PFA-based concrete [10,71].

Figure 6:Expansion of low/high PFA-based concrete samples in Na2SO4 solution [10,71].
A sustainability study was conducted on low/ high 30% and 40% PFA composites with 300Kg/m3 and 400kg/m3 OPC in 5% Na2SO4 solution for 24 months. The study observed that 30% low fly ash with 400kg/m3 OPC performed better in all compressive strength and expansion testing on cylinders/ prisms, followed by the performance of 30% high PFA with 300kg/m3. OPC concrete performed the worst and was significantly damaged after ten months of immersion. The pozzolanic reaction of PFA to absorb excessive portlandite and to convert it into C-S-H gel resulted in better performance of PFA composites in the lesser formation of ettringite, whereas a high dosage of PFA resulted in the excess formation of Si(OH)4 which exhibits swelling characteristics resulting into expansion/ cracking/ propagation of sulphate attack. Therefore, it can be elucidated that up to 30% PFA with OPC can provide better durability in the sulphate environment, as shown in Figures 5 & 6; [10,71].
The study of the impact of sulphate attack on binary concrete and ternary concrete using PFA, GGBS, and PFA/GGBS mixes as SCMs with OPC in 5% sodium sulphate, 5% magnesium sulphate and 2.5% sodium/ 2.5% magnesium sulphate solution for 270 days immersion, observed that ternary concrete mixes with up to 30% PFA+GGBS exhibited good performance as compared to individual/ binary composites of OPC with PFA and GGBS [48]. The maximum compressive strength was demonstrated with 5% PFA and 15 % GGBS binary blends, and 3.75% PFA+3.75% GGBS ternary blend. However, the complete replacement of 30 % SCMs also gave some advantages, e.g., good resistance against sulphate attack, less permeability and water absorption compared to the control mix. Still, the disadvantage is a reduction in compressive strength. Visual observations showed the worst degradation with 30% PFA followed by 30% GGBS, whereas the ternary blend of 30% PFA/GGBS showed minimum degradation. The maximum degradation was infused with a mixed solution of 2.5% sodium/ 2.5% magnesium sulphate, followed by 5% magnesium sulphate and 5% sodium sulphate. The sulphate attack by sodium sulphate is characterised by elongation, whereas loss of strength is pronounced more in MgSO4 and Na2SO4+MgSO4 [61]. The binary PFA and GGBS mixes with maximum compressive strength exhibited the lowest elongation, whereas the ternary blend showed maximum extension. However, the ternary mixture exhibited higher performance against sulphate attack in 5% magnesium sulphate and 2.5% sodium and 2.5% magnesium sulphate solution (Figure 7). The reduced elongation in SCMs mixes is due to the pozzolans’ pore-filling capability, which prevents the formation of secondary ettringite and deep propagation of cracks/ expansion. Mixing SCMs up to 30% reduced alite and celite, resulting in reduced production of portlandite, ettringite and monosulphate aluminates with SO3, thus reducing the vulnerability of concrete composites against sulphate attacks [48,62].
Figure 7:Percentage elongation of mixes with max replacement @ 30% PFA, 30% GGBS and 30% PFA+GGBS [48].

An experimental study on the impact of blending silica fume with cement concrete to improve sulphate resistance, elucidated the use of 0 % (control mix), 10%, 15% and 25% SF with class I and class V cement (350kg/m3 and 450kg/m3) with 0.4 and 0.5w/c ratios. It was elucidated that 10% SF used with OPC 450kg/m3 and 0.4w/c ratios exhibited the best performance when subjected to a concentrated sulphate attack of 5% MgSO4 for 224 and 700 days, as shown in Figure 8; [69]. The research on existing literature supports the use of pozzolans in cement concrete and geopolymer concrete for enhancement of mechanical properties and resistance of composites against sulphate attack due to absorption of portlandite, production of more C-S-H gel and filling of voids to prevent the propagation of cracks and formation of ettringite as discussed in the section above.
Figure 8:Compressive strength loss after sulphate attack of 224 and 700 days [69].

Figure 9:Reduction in compressive strength after 15 weeks of immersion in 5% MgSO4 solution [51].

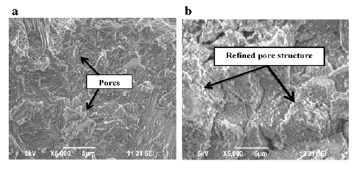
Metakaolin (MK) can be used as a pozzolan in partial cement replacement to develop a concrete composite. The researchers observed that an optimum value of 10% MK in OPC exhibited higher resistance against sulphate and chloride attacks [51]. Use of 10-15% MK exhibited up to 15% reduction in emission of CO2 [59], and 5% use of MK improved compressive strength by 10%. The compressive strength was reduced with increased use of metakaolin up to 10% and 15% MK; water absorption was observed maximum in the control mix with 0% MK and minimum in MK10%. The durability testing after 15 weeks of immersion in 5% MgSO4 solution exhibited a maximum reduction in compressive strength in control samples (MK 0%), whereas 10% MK performed the best with minimum reduction in strength and minimum water absorption in sulphate solution (Figure 9 & 10); [51]. The microstructural investigation using SEM (Figure 8) supported the findings by showing improvement in the pore structure due to pozzolanic filler capability and reduction in the formation of ettringite, thaumasite, gypsum and brucite (resultant products due to sulphate attack) in MK10% mix as compared to MK 0% [51].
Figure 10:SEM images showing improvement in pore structure due to MK10% (b) filler capability [51].
Applications of agriculture-based pozzolans like RHA and CCA
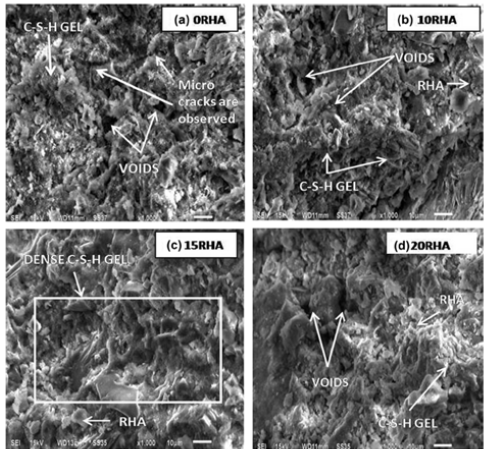
A study on the use of rice husk ash (RHA) as an agricultural pozzolanic material, elucidated the blending of 0-20% RHA with OPC as SCM and observed an increase of 25-36% in compressive strength and split tensile strength with 15% RHA at 7, 28 and 56 days of curing (Figure 11); [49]. RHA was found to be a feasible alternative pozzolan and filled the pores befittingly to reduce permeability. The rapid chloride permeability tests were performed and exhibited that RHA reduces porosity, thus exhibiting reduced chloride permeability [85]. The increase in strength was attributed to more formation of C-S-H gel due to pozzolanic reaction in cement concrete composites [49]. The microstructural analysis of RHA mixes by SEM was carried out and explained the formation of dense C-S-H gel and improvement in the void filling, with the use of up to 15% RHA in concrete composites; however, due to the formation of Si(OH)4 (because of excess SiO2 and its hydration with portlandite in aqueous solution in pozzolanic material). C-S-H gel was observed to reduce/thin out beyond 15% use of RHA, as is evident by lower strength and decreased permeability observed with 20% use of RHA (Figure 12); [49].
Figure 11:Compressive/ split tensile strength of 0-20% RHA [49].

Figure 12:Microstructural analysis of RHA mixes by SEM (a) 0% RHA, (b) 10% RHA, (c) 15% RHA, (d) 20% RHA. Thedensest formation of C-S-H gel was observed with 15% RHA (c) [49].
The researchers used Corn Cob Ash (CCA) as SCM in cement concrete using 0-30% replacement and suggested 7.5% as an optimum value for better compressive strength (Table 2); [50]. The cubes from the control mix (0% CCA) and 7.5% were immersed in 5% NA2SO4, 5% MgSO4 and 2.5%Na2SO4+2.5%MgSO4 solutions for 270 days. It was observed that the use of CCA exhibited good performance versus the control mix against all sulphate attacks in all three solutions because of the pozzolanic reaction of CCA in concrete hydration by reacting with excess Ca(OH)2, and forming more C-S-H gel, reduction of secondary ettringite and behaving as filler material to reduce the porosity of CCA blended concrete composite [50]. The 7.5% CCA blended mix exhibited reduced elongation in 5% NA2SO4 and 2.5% Na2SO4+2.5% MgSO4 solutions compared to the control mix. However, the control mix performed better in the MgSO4 solution than the 7.5% CCA mix. It exhibited lesser elongation because the control has more compressive strength than CCA mixes [50]. The strength deterioration factors were calculated for these samples using the formulae SDF = ((fcw’ – fcs’)/fcw’) x 100 (where fcw’ is the compressive strength of control specimen cubes and fcs’ is the compressive strength of sulphate immersed specimen cubes.). The control was observed to be the least impacted by sodium sulphate due to its higher compressive strength, but 7.5% CCA blended composite performed better in 5% MgSO4 and 2.5% Na2SO4+2.5% MgSO4, concluding that the use of CCA up to 7.5% is a feasible option against sulphate attacks as shown in Figure 13; [50].
Table 2:Compressive strength of 0, 5, 7.5, 10, 15, 20, 25, and 30% CCA blended cement composites [50].

Figure 13:SDF of 0% and 7.5% CCA blended cement composites immersed in 5% Na2SO4, 5% MgSO4 and 2.5% Na2SO4+2.5% MgSO4 solutions after nine months [50].

Use of natural pozzolan like zeolite
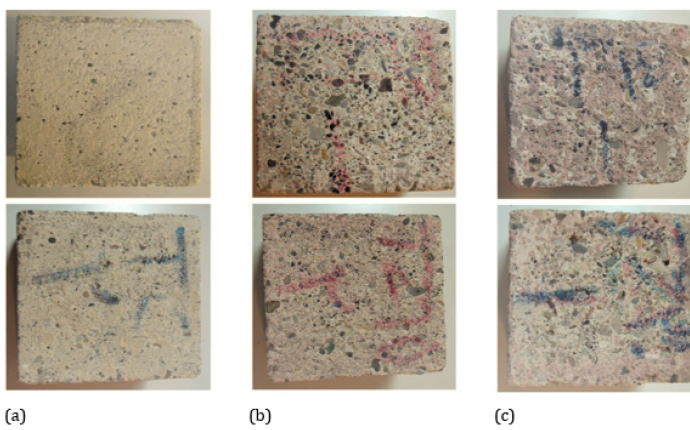
Zeolite is used effectively in the cement industry to reduce CO2 gas emissions in developed countries. Zeolite is found naturally in volcanic and sedimentary rocks and contains hydrated aluminosilicates of alkali/ alkaline metal cations [63]. These cations react swiftly with portlandite to form dense C-S-H gel. The threedimensional molecular structure of zeolite makes it a suitable filler to decrease porosity and create a defence against sulphate/chloride attack [52]. The researchers conducted an experimental study on the durability properties of concrete containing 0, 15% and 30% zeolites as SCM with OPC. The use of zeolite exhibited improvement in concrete’s mechanical and micro-structural properties. Although the compressive strength remained lower than the control mix, later curing ages showed a reduced gap in strength achievement due to a slow pozzolanic reaction [52]. The water absorption, porosity, dry shrinkage and corrosion rate were improved using the increased quantity of zeolite [52]. However, enhanced resistance against sulphate attack was observed with increased zeolite use versus the control mix (Figure 14 & 15); [52]. Using up to 30% of zeolite can decrease CO2 emissions by 30% while giving compatible structural outputs at par with OPC. However, a 15% replacement ratio produces the optimum blended composite [52]. Considering its environmental benefits and the improvements in mechanical properties of the zeolite-cement composite, it can be recommended as a suitable pozzolanic SCM [52].
Figure 14:Weight changes in 0%, 15%, and 30% zeolite mixes after immersion in H2SO4 for 300 days [52].

Figure 15:Comparison of surface degradation in 0% (a), 15% (b), and 30% (c) zeolite mixes [52].
Application of SF, PFA and GGBS in geopolymer-based concrete for better sulphate resistance
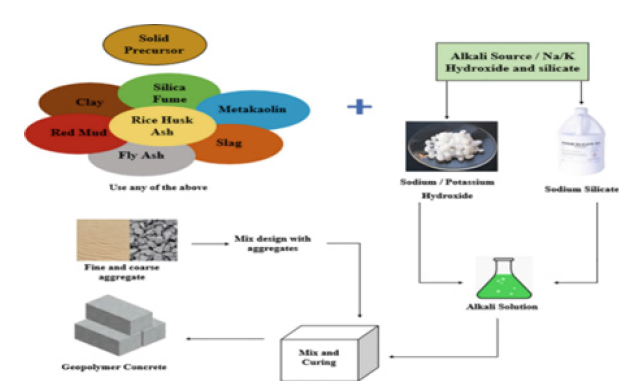
The use of SF, PFA and GGBS in manufacturing geopolymerbased concrete is already in practice due to their enhanced environmental benefits. Their use as total cement replacement to produce geopolymer concrete is explained in Figure 16; [67], where industrial/ agricultural/ natural pozzolans are mixed with an alkali activator with normal sand and aggregates to produce geopolymer concrete [67].
Figure 16:Manufacturing process of geopolymer concrete [67].
A study was conducted on geopolymer concrete containing 100% PFA, 50% PFA+50% GGBS and 100% GGBS. The research found that 100% GGBS geopolymer concrete performed at par with OPC concrete. The study exhibited 57.6MPa compressive strength on 28 days, followed by 50% PFA+50% GGBS composite achieving 52.5MPa strength, and 100% PFA geopolymer provided the least compressive strength of 11 MPA as shown in Figure 17; [68]. The sulphate resistance studies were conducted by immersing geopolymer concrete cubes in 3% H2SO4 for 28 days. The maximum loss in weight and compressive strength postacid attack was observed in 100% PFA composite by 5.6% and 33.6%, respectively. Whereas 100% GGBS geopolymer composite performed the best with the least weight and compressive strength reduction by 1.5% and 11%, respectively, as shown in Table 3. The GGBS-based geopolymers exhibited a par performance with OPC concrete because GGBS is considered a suitable/complete cement replacement [68].
Figure 17:Compressive strength (MPa) 7 and 28 days [68].

Table 3:Comparison of loss in weight and compressive strength of geopolymer concrete composites after the acid attack [68].

Conclusion
Based on the detailed literature review and study of different
experimental works, this paper suggests the following conclusions:
1) The evolution and improvement of construction materials is a
continuous process, and more avenues are required to explore
to produce environmentally friendly, cost-effective, and robust
materials.
2) Cement is the most used construction material and produces
around a ton of CO2 per ton of cement during manufacturing
which results in the industry emitting up to 10% of global CO2
emissions.
3) The hydration of cement involves the production of the two
most pronounced compounds, i.e., Ca(OH)2 and C-S-H gel.
The C-S-H gel is responsible for the strength of concrete
and is produced by the hydration of C3S, C2S and pozzolans.
Portlandite is responsible for an anti-corrosion alkaline
environment. Still, it causes a reduction in strength by reacting
with reactive metal cations on sulphate attack and converts to
and is produced by the hydration of C3S, C2S and pozzolans.
gypsum and brucite.
4) The internal sulphate attack is caused by gypsum and ettringite,
produced during the hydration of cement. The external attack
is severe and occurs over an extended period by absorption
of sulphate solutions from the atmosphere and soil water,
converts monosulphate aluminate hydrates into ettringite and
causes expansion and cracks leading to ultimate failure.
5) The concentration of sulphate solution, permeability, period
of exposure and cement composition influence the degree of
sulphate attack/ deterioration.
6) The water-cement (w/c) ratio of more than 0.45 makes the
concrete susceptible to external sulphate attack. In contrast, a
w/c ratio of 0.35 and less is found to be the least vulnerable to
external sulphate attack even after decades of exposure.
7) Using pozzolans as SCMs is considered beneficial in reducing
CO2 emissions and improving strength by producing more
C-S-H gel on the chemical reaction of silicates and portlandite.
8) The use of high sulphate cement and blending of various
pozzolanic materials improve the sustainability of cement
concrete composites against sulphate attacks.
9) The microstructural studies using SEM and XRD support the
use of pozzolanic composites to improve pore structures and
the formation of lesser ettringite during sulphate attack.
References
- Nadir HM, Ahmed A (2020) Causes and monitoring of delays and cost overrun in construction projects in Pakistan. International Journal of Engineering Invention 9(8): 20-33.
- The Editors of Encyclopaedia (2020) Britannica.
- Rehan R, Nehdi M (2005) Carbon dioxide emissions and climate change: Policy implications for the cement industry. Environmental Science & Policy 8(2): 105-114.
- Worrell E, Price L, Martin N, Hendriks C, Meida LO (2001) Carbon dioxide emissions from the global cement industry. Annu Rev Energy Environ 26: 303-329.
- (2018) If the cement industry were a country, it would be the third-largest emitter in the world.
- Rajkumar MR (2017) Recent advances in materials, mechanics and management. Proceedings of the 3rd International Conference on Materials, Mechanics and Management, Trivandrum, India, p. 450.
- Vashisht P, Paliwal MC (2020) Partial replacement of cement with rice husk ash in cement concrete. International Journal of Engineering Research & Technology 9(12): 322-325.
- Ahmed A, Hyndman F, Kamau J, Fitriani H (2020) Rice husk ash as a cement replacement in high strength sustainable Concrete. Materials Science Forum 1007: 90-98.
- Nadir HM, Ahmed A (2021) Comparative evaluation of potential impacts of agricultural and industrial waste pozzolanic binders on strengths of concrete. Journal of Material Sciences & Manufacturing Research 2(2): 1-8.
- Nadir HM, Ahmad A (2022) The mechanisms of sulphate attack in concrete–A review. Mod App Matrl Sci 5(2): 658-670.
- Yildirim G, Sahmaran M, Ahmed HU (2015) Influence of hydrated lime addition on the self-healing capability of high-volume fly ash incorporated cementitious composites. Journal of Materials in Civil Engineering 27(6).
- Qadir HH, Faraj RH, Sherwani AFH, Mohammed BH, Younis KH (2020) Mechanical properties and fracture parameters of ultra-high performance steel fibre reinforced concrete composites made with extremely low water per binder ratios. SN Applied Sciences 2: 1594.
- Shahbazpanahi S, Faraj RH (2020) Feasibility study on the use of shell sunflower ash and shell pumpkin ash as supplementary cementitious materials in concrete. Journal of Building Engineering 30: 101271.
- Shahbazpanahi S, Tajara MK, Faraj RH, Mosavi A (2021) Studying the C-H crystals and mechanical properties of sustainable concrete containing recycled coarse aggregate with used nano-silica. Crystals 11(2): 122.
- Abbasi S, Jannaty MH, Faraj RH, Shahbazpanahi S, Mosavi A (2020) The effect of incorporating silica stone waste on the mechanical properties of sustainable concretes. Materials 13(17): 3832.
- Onuaguluchi O, Panesar DK (2014) Hardened properties of concrete mixtures containing pre-coated crumb rubber and silica fume. Intl J of Clean Prod 82: 125-131.
- Najimi M, Sobhani J, Ahmadi B, Shekarch M (2012) An experimental study on durability properties of concrete containing zeolite as a highly reactive natural pozzolan. Construction and Building Materials 35: 1023-1033.
- Mehta PK (1999) Concrete technology for sustainable development. Concr Int 21(11): 47-52.
- Damtoft JS, Lukasik J, Herfort D, Sorrentino D, Gartner EM (2008) Sustainable development and climate change initiatives. Cem Concr Res 38(2): 115-127.
- Ahmed A, Nadir H, Colin Y, Lee Y (2020) Use of Coconut COIR in Fibre Reinforced Concrete. Int Journal of Modern Approaches on Material Science 3(4): 391-399.
- Anon (2022) Bogues compounds: Know 4 types of major components of cement quickly. Civil Giant.
- Khan F (2020) Chemical composition of cement. Construction How.
- Show D (2020) Cement: Definition, introduction, types, composition and tests. Mechanical Notes.
- Prodyogi (2018) Bogue compounds: Hydration reactions.
- Anon (2022) What is hydration of cement? know heat of hydration & products of cement hydration here. Civil Giant.
- Nadir HM, Ahmed A (2021) Comparative evaluation of potential impacts of agricultural and industrial waste pozzolanic binders on strengths of concrete. Journal of Material Sciences & Manufacturing Research 2(2): 1-8.
- Johannes F (2021) Chemistry of setting. Petroleum Engineer's Guide to Oil Field Chemicals and Fluids (3rd edn), Sceinece Direct, Amsterdam, Netherlands.
- Shweta G, Devender S (2020) CO2 sequestration on cement. Start-Up Creation (2nd edn), Woodhead Publishing Series in Civil and Structural Engineering, pp. 109-142.
- Hydration of portland cement.
- Sidney M, Francis Y (1981) Concrete. Prentice-Hall Inc, New Jersy, USA, p. 671.
- Steven HK, William CP, Kerkhof B (1988) Design and control of concrete mixes. Portland Cement Association, Skokie, USA, p. 205.
- Michael SM, John JZ (1999) Materials for civil and construction engineers. Addison Wesley Longman, USA. In: Jeremy PI (Ed.), (2013) Geomaterials under the microscope, pp. 75-120.
- Vikas (2019) Hydration of Cement. Scribd, p. 8.
- Eldidamony H, El-Sokkari TM, Khalil KA, Heikal M, Inas A (2012) Hydration mechanisms of calcium sulphoaluminate C(4)A(3)(S)over-bar, C(4)A(S)over-bar phase and active belite beta-C2S. Ceramics Silikaty 56(4): 389-395.
- Cefis N, Claudia C (2017) Chemo-mechanical modelling of the external sulfate attack in concrete. Cement and Concrete Research 93: 57-70.
- Lei M, Peng L, Shi C, Wang S (2013) Experimental study on the damage mechanism of tunnel structure suffering from sulfate attack. Tunnelling and Underground Space Technology 36: 5-13.
- Neville A (2004) The confused world of sulfate attack on concrete. Cement and Concrete Research 34(8): 1275-1296.
- Collepardi M (2003) A state-of-the-art review on delayed ettringite attack on concrete. Cement and Concrete Composites 25(4-5): 401-407.
- Anon (2022) Sulphate attack on concrete | how to prevent sulphate attack on concrete? Civil Giant.
- Ahmed A, John K (2017) Performance of ternary class f pulverised fuel ash and ground granulated blast furnace slag concrete in sulfate solutions. European Journal of Engineering Research and Science 2(7): 8.
- Liu K, Mo L, Deng M, Tang J (2015) Deterioration mechanism of Portland cement paste subjected to sodium sulfate attack. Advances in Cement Research 27(8): 477-486.
- Idiart AE, Lopez CM, Carol I (2011) Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: A meso-scale model. Cem Concr Compos 33(3): 411-423.
- Marchand J, Older I, Skalny JP, (2003) Sulfate Attack on Concrete. CRC Press, USA, p. 232.
- Santhanam M, Cohen MD, Olek J (2002) Mechanism of sulfate attack: A fresh look: Part 1: Summary of experimental results. Cem Concr Res 32(6): 915-921.
- Stutzman PE, Bullard JW, Feng P (2016) Phase analysis of Portland cement by combined quantitative X-Ray powder diffraction and scanning electron microscopy. Journal of Research of the National Institute of Standards and Technology 121: 1-61.
- Zhang T, Vandeperre LJ, Cheeseman CR (2014) Formation of Magnesium Silicate Hydrate (M-S-H) cement pastes using sodium hexametaphosphate. Cement and Concrete Research 65: 8-14.
- Sotiriadis K, Nikolopoulou E, Tsivilis S (2012) Sulfate resistance of limestone cement concrete exposed to combined chloride and sulfate environment at low temperature. Cement and Concrete Composites 34(8): 903-910.
- Ahmed A, Kamau J (2017) Performance of ternary class f pulverised fuel ash and ground granulated blast furnace slag concrete in sulfate solutions. European Journal of Engineering Research and Science 2(7): 8.
- Divya C, Rafat S, Kunal (2015) Strength, permeability and microstructure of self-compacting concrete containing rice husk ash. Biosystems Engineering 130: 72-80.
- Kamau J, Ahmed A, Hirst P, Kangwa J (2016) Suitability of corncob ash as a supplementary cementitious material. International Journal of Materials Science and Engineering 4(4): 215-228.
- Kavitha OR, Shanthi VM, Arulraj GP, Sivakumar VR (2016) Microstructural studies on eco-friendly and durable Self-compacting concrete blended with metakaolin. Applied Clay Science 124-125: 143-149.
- Najimi M, Sobhani J, Ahmadi B, Shekarchi M (2013) An experimental study on durability properties of concrete containing zeolite as a highly reactive natural pozzolan. Construction and Building Materials 35: 1023-1033.
- Ideker C, Thomas MDA, Farshad R, Eric G, Cyrille D (2015) Alkali–silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cement and Concrete Research 76: 130-146.
- Powers TC, Steinour HH (1955) An interpretation of some published researches on the alkali-aggregate reaction part 1-the chemical reactions and mechanism of expansion. J Am Concr Inst 6: 497-516.
- (2022) Sulphate Resistant Cement. Standards and Specifications, SRC Grade, UAE.
- McCarthy MJ, Dyer TD (2019) Pozzolanas and Pozzolanic Materials. Lea’s Chemistry of Cement and Concrete 363-467.
- Wang J, Cai G, Wu Q (2018) Basic mechanical behaviours and deterioration mechanism of RC beams under chloride-sulphate environment. Construction and Building Materials 160: 450-461.
- Yuan Q, Liu Z, Zheng K, Ma C (2021) Inorganic cementing materials. Civil Engineering Materials 17-57.
- Cassagnabère F, Mouret M, Escadeillas G, Broilliard P, Bertrand A (2010) Metakaolin, a solution for the precast industry to limit the clinker content in concrete: Mechanical aspects. Construction and Building Materials 24(7): 1109-1118.
- Nehdi M, Pardhan M, Koshowski S (2004) Durability of self- consolidating concrete incorporating high-volume replacement composite cements. Cement and Concrete Research 34(11): 2103-2112.
- Bapat JD (2012) Mineral admixtures in cement and concrete: CRC Press.
- Moon HY, Lee ST, Kim SS (2003) Sulphate resistance of silica fume blended mortars exposed to various sulphate solutions. Canadian Journal of Civil Engineering 30(4): 625-636.
- Colella C (2007) In: Cˇejka J, Bekkum HV, Corma A, Schueth F (Eds.), Introduction to zeolite science and practice. Amsterdam, Elsevier, pp. 999-1035.
- Ahmaran M, Özkan N, Keskin SB, Uzal B, Yaman IÖ, et al. (2008) Evaluation of natural zeolite as a viscosity-modifying agent for cement-based grouts. Cem Concr Res 38(7): 930-937.
- Parashar AK, Sharma P, Sharma N (2022) Effect on the strength of GGBS and fly ash based geopolymer concrete. Materials Today: Proceedings.
- Xie J, Zhao J, Wang J, Wang C, Huang P, et al. (2019) Sulfate resistance of recycled aggregate concrete with GGBS and fly ash-based geopolymer. Materials (Basel) 12(8): 1247.
- Thomas BS, Yang J, Bahurudeen A, Chinnu SN, Abdalla JA, et al. (2022) Geopolymer concrete incorporating recycled aggregates: A comprehensive review. Cleaner Materials 3: 100056.
- Jawahar J, Lavanya D, Sashidhar, Chundpalle (2016) Performance of fly ash and GGBS based geopolymer concrete in acid environment. International Journal of Research and Scientific Innovation 3: 101-104.
- Ahmed D, Elwahab A, Awad M, Elyamany, Hafez, et al. (2008) Magnesium sulfate resistance of silica fume concrete specimens and r.c columns.
- Higgins DD (2003) Increased sulfate resistance of ggbs concrete in the presence of carbonate. Cement and Concrete Composites 25(8): 913-919.
- Torii K, Taniguchi K, Kawamura M (1995) Sulfate resistance of high fly ash content concrete. Cement and Concrete Research 25(4): 759-768.
- Tixier R, Mobasher B (2003) Modeling of damage in cement-based materials subjected to external sulfate attack I: Formulation. ASCE J Mater Civ Eng 15(4): 305-313.
© 2022 Ash Ahmed. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






