- Submissions

Full Text
Perceptions in Reproductive Medicine
Early Ovarian Failure Reversed: A Justification for the Myasthenia Gravis Diagnosis
Ghazal K1,2,3*, Azzi J4, Chatila AR5 and Hassan JE1,3,6
1Assistant Professor Obstetrics and Gynecology Department, Lebanese University, Faculty of Public Health, Italy
2Obstetrics and Gynecology Department, Al Zahraa Hospital University Medical Centre, Italy
3Obstetrics and Gynecology Department, Rafik Hariri Hospital University Medical Center, Italy
4Assistant Professor, Pharmacist Department, Lebanese University, Faculty of Public Health, Italy
5Clinical Assistant Professor Clerkship Director in Neurology, Lebanese American University, Italy
6Head of Obstetrics and Gynecology Department, Al Zahraa Hospital University Medical Centre, Italy
*Corresponding author:Kariman Ghazal, Obstetrics and Gynecology Department, Lebanese University, Faculty of Public Health, Italy
Submission: August 15, 2023;Published: August 28, 2023

ISSN: 2640-9666Volume5 Issue5
Abstract
Premature Ovarian Failure (POF) is defined as anovulation with amenorrhoea in females younger than 40 years with evidence of hypo-oestrogenic and hypergonadotropic serum levels. Recognised as being of autoimmune aetiology, there have been, however, very few reports on POF in conjunction with MG. We describe a case of Premature ovarian failure, and the reason for the Myasthenia Gravis diagnosis and perform a brief literature review.
Keywords:Ovary; Myasthenia graves; Amenorrhea; Ultrasound; Thymic enlargement acetylcholine
Abbreviations: POF: Premature Ovarian Failure; FSH: Follicle-Stimulating Hormone; LH: Luteinizing Hormone; RNS: Repetitive Nerve Stimulation
Introduction
After puberty and before the age of 40, Premature Ovarian Failure (POF), which is characterized by high levels of the Follicle-Stimulating Hormone (FSH), the Luteinizing Hormone (LH), and low estrogen levels, causes an unphysiological termination of menstruation. Research suggest that 0.1% of secondary amenorrhea patients will get POF before the age of 30 [1]. We herein describe a case of POF, and the reason for the Myasthenia Gravis diagnosis is a causative connection with a MG autoimmune origin. The aim of this study is to improve the level of diagnosis and treatment of such problems by clinicians.
Case Report
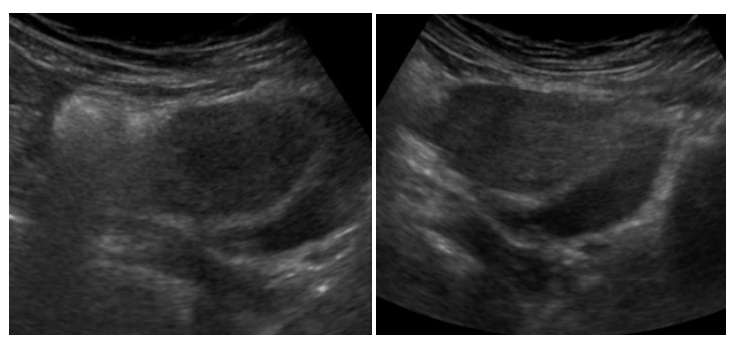
In June 2017, an undiagnosed case of bilateral thigh weakness, right eyelid droop, and double vision were given by a 21-year-old nulliparous woman. The patient presented the mildest and most severe symptoms during the morning and evening, which grew worse with exhaustion and disappeared after rest. The individual continued to complain, nevertheless, of paralysis and evident limb weakness after the activity. She had a normal visual field and bilateral partial ptosis. Additionally, the individual suffered amenorrhea for six months prior to developing limb weakness. Her menses had been regular ever since she reached menarche at the age of 14. The patient was found to have typical secondary sexual characteristics and no signs of vitiligo, hyperpigmentation, or hypothyroidism. As a result, the patient visited many specialists in quick succession after her gynecologist recommended seeing a neurologist for a diagnosis after noticing uterine shrinkage on a pelvic ultrasound (Figure 1 & 2). The patient was nonetheless identified as having MG. The individual also had no family history of any inherited illnesses, or alcohol use but was a severe smoker. General blood test was normal, but she had hypo-estrogenemia with a post-menopausal range of Follicle-Stimulating Hormone (FSH) 65mIU/ml and Luteinizing Hormone (LH) levels 97.3 mIU/ml>20. Antibodies to the AChR were detected largely increased 100.0nmol/l N<0,2 . Computed Tomography (CT) of the chest and MRI revealed thymic enlargement. Her Single-Fiber Electromyography (SFEMG) showed prolonged jitter and Repetitive Nerve Stimulation (RNS) revealed a significant decremental response. The muscle power of both hips was 3/5.
Figure 1&2:Pelvic ultrasound showed uterus atrophic, with ovaries reduced in volume.
The patient was admitted, she received diphenhydramine with Rituximab infusion, parenteral nutrition, and symptomatic treatment. Myasthenic crisis gradually resolved. Maintenance treatment of pyridostigmine bromide 60mg/8 hours. Menstrual cycle was back to normal with HRT treatment. The patient underwent a thymectomy in April 2019 and was monitored until December 2020-2021. After this period the patient stopped any medication was back to normality.
Discussion
Human ovaries have long been recognized as a target for autoimmune attacks leading to ovarian dysfunction, especially Premature Ovarian Failure (POF). Up to 20% of patients [1,2]. For our patient, we propose that the POF was induced by an autoimmune attack on the ovaries. This hypothesis is also strengthened by previous reports showing that autoimmunity is present in 18-92% of POF patients who have anti-ovarian antibodies AOA or other autoantibodies. This combination of MG and POF is interesting since impaired immunoregulatory mechanisms could contribute to both diseases [3]. In our case, from clinical symptoms and gynecological history we diagnosed MG. The high presence of autoantibodies Against The Acetylcholine Receptor (AChR) fixed diagnosis because these are detected in up to 90% of MG patients. Other antibodies found in MG are directed to Muscle-Specific Receptor Tyrosine Kinase (MuSK), which is a transmembrane component of the postsynaptic neuromuscular junction, only a minority of MG patients are seronegative like our case. It has been hypothesized that AChR could exist in the ovaries, and that cross-reactivity with antibodies to the muscles and AChR could explain POF associated with MG [2,3]. Autoimmunity is further strengthened by the presence of Anti-Ovarian Antibodies (AOA) found in three patients with MG and POF [3,4]. Interestingly, in our literature review, one patient was found to have autoantibodies directed to the folliclestimulating hormone, and another patient had autoantibodies against the luteinizing hormone [1,2]. AOA were not tested in our case as the test is not available in our country, which is a limitation of our study.
Our case presented secondary amenorrhea preceding MG. She resumed her menses after thymectomy, which accords with the cases published by Çakir [5,6].This indicates that the thymus might play a role in the pathogenesis of both conditions, which are already known to be a source of driving autoimmunity. AChR autoantibodies are thought to originate from the hyperplastic germinal centers in the thymus. This could well explain why most of the reported cases, including our case, are AChR positive where a causal link is observed. Moreover, an increased expression of estrogen receptor α has been found on thymocytes and peripheral T lymphocytes in MG patients; this dysregulation might influence the progression of the autoimmune response for POF [5,7]. The treatment with 17β-estradiol enhanced the production of anti- AChR-Ab in experimental autoimmune MG [7], which implies that estrogen might contribute to the aggravation of MG but none in our case. An acetylcholinesterase inhibitor is the mainstay of medical treatment, however, people who are resistant to it may also benefit from prednisone or plasmapheresis. An improvement in symptology is correlated with a quantitative decrease in antibody titer [8].
In conclusion, there is a causal link between MG and POF, the occurrence of which is not purely coincidental. The presence of autoantibodies, and the resolution of diseases after thymectomy or immunotherapy in some cases. Large-scale studies are required in order to provide better insights into the pathogenesis of both these diseases [9,10].
Author Contributions
Dr kariman with Dr Jihad are responsible for the conception and design, acquisition of data, analysis and interpretation of data, drafting the article, and gave final approval to the version to be submitted. Dr joelle Abdul contributed by revising it critically for important intellectual content.
References
- Ryan MM, Jones HR (2004) Myasthenia gravis and premature ovarian failure. Muscle Nerve 30(2): 231-233.
- Ebrahimi M, Asbagh FA (2015) The role of autoimmunity in premature ovarian failure. Iran J Reprod Med 13(8): 461-472.
- Williamson H, Phansey S, Mathur S, Mathur RS, Baker ER, et al. (1980) Myasthenia gravis, premature menopause, and thyroid autoimmunity. Am J Obstet Gynecol 137(8): 893-901.
- Komorowska B (2016) Autoimmune premature ovarian failure. Prz Menopauzalny 15(4): 210-214.
- Çakır ED, Özdemir Ö, Eren E, Sağlam H, Okan M, et al. (2011) Resolution of autoimmune oophoritis after thymectomy in a myasthenia gravis patient. J Clin Res Pediatr Endocrinol 3(4): 212-215.
- Arifin NM, Khoo CS, Leong D, Tee TY, Tan HJ (2021) Myasthenia gravis and premature ovarian failure-a causal link. Neurol Neurochir Pol 55(2): 233-235.
- Nancy P, Aknin SB (2005) Differential estrogen receptor expression in autoimmune myasthenia gravis. Endocrinology 146(5): 2345-2353.
- Liu X, Li R, Jing Y (2018) Resolution of primary ovarian insufficiency after corticosteroid administration in a myasthenia gravis patient: Report and minireview of the literature. Neurol India 66(6): 1807-1810.
- Cao L, Liu W, Zhu Z (2019) Clinical characteristics and relationship between myasthenia gravis and premature ovarian failure: Report of two cases. J Int Med Res 47(8): 3992-3997.
- AlAsiri SA (2020) Primary ovarian insufficiency and myasthenia gravis. Pak J Med Sci 36(3): 586-587.
© 2023 Ghazal K. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






