- Submissions

Full Text
Progress in Petrochemical Science
Utilization of NaOH Treated Rice Husk for Adsorptive Discharge of Eriochrome Black-T from an Aqueous Solution
Muhammad Imran Khan1*, Abdallah Shanableh1 and Suryyia Manzoor2
1Research Institute of Sciences and Engineering (RISE), University of Sharjah, United Arab Emirates
2Institute of Chemical Sciences, Bahauddin Zakariya University, Pakistan
*Corresponding author:Muhammad Imran Khan, Research Institute of Sciences and Engineering (RISE), University of Sharjah, United Arab Emirates
Submission: February 01, 2024;Published: February 23, 2024

ISSN 2637-8035Volume6 Issue1
Abstract
This article reports batch adsorption of Eriochrome Black-T (EBT) dye from an aqueous solution onto NaOH Treated Rice Husk (TRH). The morphology of untreated and treated rice husk was studied by Scanning Electron Microscopy (SEM). Adsorption of EBT from an aqueous solution onto TRH was demonstrated by using Fourier Transform Infrared (FTIR) spectroscopy. The effect of operational parameters on the percentage removal of EBT from an aqueous solution and adsorption capacity was investigated in detail. The adsorptive removal of EBT from aqueous solution was increased with contact time, mass of TRH and temperature while declined with initial concentration of EBT aqueous solution. Similarly, adsorption capacity was enhanced from 0.71 to 1.61mg/g, 1.61 to 17.0mg/g and 1.41 to 1.68mg/g with increase in contact time, initial concentration of EBT aqueous solution and temperature respectively while decreased from 6.17 to 1.61mg/g with increase in mass of TRH. Adsorption kinetic study showed that adsorption of EBT onto TRH fitted well to pseudo-second-order kinetics because the value of correlation coefficient (R2= 0.996) was close to unity. Adsorption isotherms including Langmuir, Freundlich, Dubinin-Radushkevich (D-R) and Tempkin were applied to experimental data. Results suggested that experimental data fitted well to Freundlich adsorption isotherm. Adsorption thermodynamics study indicated that adsorption of EBT onto Treated Rice Husk (TRH) was endothermic process because the value of enthalpy (ΔHo= 20.57KJ/mol) was positive for it. Moreover, the negative values of Gibb’s free energy indicated that adsorption of EBT onto TRH was spontaneous process.
Keywords:Eriochrome Black-T (EBT); Endothermic process; Treated Rice Husk (TRH); Adsorption; Pseudo-second-order model
Introduction
Azo dyes released from several textile industries [1], leather industries [2], food industries [3], paint [4], and pigment manufacturing [5] industries and at least 100,000 different types of dye [6] that are commercially obtainable presently result gloomy influence on the environmental systems because of their strong aromatic [7] complex structure. Dyes block the perception of sunlight into water bodies due to this complexity which results to decline in aquatic-photosynthetic activity and growth of bacteria. Therefore, it can be resistant to normal biodegradation. Due to carcinogenic, mutagenic and teratogenic properties as well as respiratory toxicity, the most dyes not only demolish aquatic organisms but also anguish humans [8]. Therefore, the adsorptive removal of synthetic organic dyestuff from waste effluents becomes environmentally important [9]. Eriochrome Black-T is one of the major carcinogenic dyes which are extremely devastating to human health. As EBT causes severe eye irritation, skin allergic irritation, when ingested, it causes digestive track disorders. Moreover, EBT when inhaled can cause respiratory tract disorders leading to cancerous effects. The treatment methods including chemical methods, physical methods and biological methods were for dye-bearing wastewater [10-15]. Among them, adsorption decolonization is the most utilized method [16]. It has been shown to be one of the most promising employed methods for the removal of both inorganic [17] and organic pollutants [18] from polluted water. It can be classified into physical adsorption and chemical adsorption based on different interaction forces between the absorbent and the adsorbate [19]. The physical adsorption was resulted by intermolecular force (Van der Waals force) [20] while the chemical adsorption was resulted by chemical bonding or surface coordination compounds by adsorbate molecules and adsorbents by means of ion exchange, electron migration and electron pair sharing [21,22]. The activated carbon adsorbents and metal and non-metal oxide adsorbents were commonly utilized adsorbents. The activated carbon is the most exemplary adsorbent. Its adsorption performance is excellent. However, the relatively higher price and lower mechanical strength are the disadvantages of the activated carbon. Hence, it is of great interest to find cheap and potent substitutes of the existing commercial activated carbon, including active aluminum molecular sieve, polymer adsorbent, silica gel and biological adsorbent and so on.
Previously, we used bio adsorbent (leaf powders of different plants) [23-26], polymeric ion exchange membranes (both commercial and synthesized anion exchange membranes) [27-30] for the removal of dyes from an aqueous solution. Moreover, we also employed bio adsorbent (rice husk) for adsorption of heavy metal ions from an aqueous solution [31-34]. Herein, we utilized NaOH Treated Rice Husk (THR) for adsorptive removal of Eriochrome Black-T (EBT) from an aqueous solution at room temperature in order to extend our work. To the best of our knowledge, adsorption of EBT onto NaOH Treated Rice Husk (TRH) has not been studied yet. As Asian countries are among the world’s major producers of rice (5.2 million tons annually) and its husk which forms 20-23% of the whole rice grain is considered as unwanted waste material that actually poses a disposal problem for mill owners. Its basic composition is proteins, cellulose, hemicellulose and lignin, containing hydroxyl and carboxyl functional groups available to interact with cations [32,34-36]. This research reports the utilization of NaOH treated rice husk as an outstanding adsorbent for the removal of Eriochrome Black-T (EBT) from an aqueous solution. The effect of contact time, mass of treated rice husk, initial concentration of EBT aqueous solution and temperature on the removal of EBT and adsorption capacity was investigated. Adsorption kinetics, equilibrium and thermodynamics for adsorption of EBT onto treated rice husk were discussed in detail.
Experimental
Materials
Eriochrome Black-T (EBT) and Sodium Hydroxide (NaOH) were bought from Sinopharm Chemical reagent Co. Ltd, Shanghai, China. All the chemicals were utilized as received. Throughout this work, deionized water was utilized. Figure 1 represents the chemical structure of EBT. The stock solution of 1000mg/L was prepared by dissolving 1.0g of accurately weighed dye into 1L of deionized water and required concentrations were obtained by further dilution of stock solution. All the chemicals used in the experiments were of analytical reagent grade. The properties of EBT are given in Table 1.
Figure 1:Chemical structure of Eriochrome black-T.

Table 1:The properties of Eriochrome Black T.

Adsorbent
The rice mill, Punjab, Pakistan kindly provided husk of basmati rice. The rice husk was thoroughly washed with distilled water to withdraw dust particle and oven dried at 80 oC till constant weight was obtained. The chemical analysis of husk was carried out by using Neutron Activation Analysis (NAA) and Atomic Absorption Spectrometry (AAS) methods for their trace metal contents and achieved results were reported [31,34]. From these results, it was observed that the quantity of metals such as Na, K, Pb and Fe were present in μg per g of sample. Silica contents were found to be 18.27 (0.62%) of TRH [33,34]. The small number of elements in TRH was studied via standard methods.
Modification of rice husk with NaOH
The dried and washed rice husk was treated with 1.0M NaOH solution. In a typical procedure, 50g of rice husk was added into NaOH solution into a 1.0L beaker. The mixture was stirred vigorously for one day. After that the rice husk was washed with distilled water until neutral pH was attained. Then, it was dried in an oven at 80 oC till constant weight was obtained. It was stored in an airtight container and named as treated rice husk.
Batch adsorption procedure
Herein, batch adsorption of Eriochrome Black T from an aqueous solution onto treated rice husk was performed as described in literature [23-27,37-43]. In a typical procedure, we prepared an aqueous solution of Eriochrome Black T by dissolving the measured amount of Eriochrome Black T into deionized water at ambient temperature. The measured amount of treated rice husk was shaken into 20mL of EBT aqueous solution at agitation speed of 140rmp. To find out the optimized contact time, the measured mass of treated rice husk was shaken into 20mL of EBT aqueous solution with initial concentration of 20mg/L for different time intervals such as 20, 24, 48, 72, 96, 168 and 216 hours to determine the optimum contact time. The optimized mass of treated rice husk was calculated by employing varying masses of the treated rice husk such as 0.04, 0.08, 0.12, 0.16 and 0.20g into 20mL of EBT aqueous solution with initial concentration of 20mg/L of EBT for 96 hours at ambient temperature. To study adsorption isotherm, the calculated amount of treated rice husk (0.20g) was shaken for 96 hours into 20mL of EBT aqueous solution with initial concentration of 20, 50, 70, 100, 150, 200, 250, 300 and 400mg/L at room temperature. Adsorption thermodynamics for adsorption of EBT onto treated rice husk was investigated by shaking treated rice husk (0.20g) into 20mL of EBT aqueous solution with initial concentration of 20mg/L at 298, 313, 323 and 333K for 96 hours at speed 140rmp. The concentration of EBT was calculated by determining the absorbance of the supernatant at wavelength (λmax.= 530nm for EBT) by employing UV/VIS spectrophotometer (UV-2550, SHIMADZU). The plot of wavelength versus absorbance for EBT to find out maximum wavelength (λmax.) is shown in Figure 2. The concentration of EBT was calculated by using calibration curve. The percentage removal of EBT from an aqueous solution by treated rice husk and adsorption capacity was measured by below relationships:
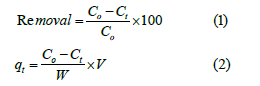
where Co and Ct indicate concentration of EBT at initial state and at time t respectively. Similarly, V shows volume of EBT aqueous solution and W denotes the mass of treated rice husk respectively.
Figure 2:The plot of wavelength versus absorbance for EBT.

Characterization
FTIR test: FTIR spectrometer (Vector 22, Bruker) having resolution
of 2cm-1 and total spectral range of 4000-400cm-1 was employed
to study TRH before and after adsorption of EBT onto it by
utilizing Attenuated Total Reflectance (ATR).
Morphological study: Morphology of untreated and NaOH
treated rice husk was investigated in detail by using field emission
scanning electron microscope (FE-SEM, Sirion200, FEI Company,
USA).
Effect of operational parameters
Effect of contact time: The influence of contact time on the
percentage removal of EBT from aqueous solution and adsorption
capacity was investigated by keeping mass of treated rice husk
(0.20g), initial concentration of EBT aqueous solution (20mg/L),
volume of EBT aqueous solution (20mL), pH= 7 and stirring speed
(140rpm) constant at room temperature.
Effect of mass of TRH: The effect of mass of treated rice husk
on the percentage removal of EBT and adsorption capacity were
explored by varying mass of it from 0.04 to 0.20g keeping contact
time (96h), volume of EBT aqueous solution (20mL), initial concentration
of EBT aqueous solution (20mg/L), pH=7 and stirring speed
(140rpm) constant at room temperature.
Effect of initial concentration of EBT aqueous solution: It
was studied by changing initial concentration of EBT aqueous solution
from 20 to 400mg/L keeping mass of treated rice husk (0.20g),
contact time (96h), volume of EBT aqueous solution (20mL), pH=7
and stirring speed (140rmp) constant at room temperature.
Effect of temperature: It was elucidated by changing temperature
from 298 to 333K keeping contact time (96 hours), volume of
EBT aqueous solution (20mg/L), mass of THR (0.20g), initial concentration
of EBT aqueous solution (20mg/L), pH=7 and stirring
speed constant (140rmp).
Effect of pH: The impact of surface modification due to variations
in solution pH was studied with in the pH range of 2-10. The
20mL aqueous solutions at optimized dye’s concentration were taken
in separate flasks and the pH of each solution was adjusted accordingly
by adding 0.1M NaOH or HCl solution. This was followed
by the addition of TRH adsorbent to every solution separately. The
mixtures were left for shaking and after a defined interval of time,
the percentage removal of dye at each pH was calculated.
Adsorption kinetics
Adsorption kinetics for adsorption of EBT from aqueous solution onto TRH was explored by using several kinetic models.
Pseudo-first-order model: The linear form of Lagergren pseudo- first-order kinetic model is given as [1-4].
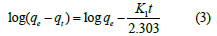
where k1 (/min), qe (mg/g) and qt (mg/g) shows rate constant of pseudo-first-order model, concentration of EBT adsorbed at equilibrium and time t respectively.
Pseudo-second-order model: The linear form of pseudo-second- order kinetic model is shown as [4,5].

where k2 (g/mg. min) is the rate constant of pseudo-second-order model.
Elovich model: It has been used for the chemisorption of gases onto heterogeneous surfaces and solid systems and has also now found an application for the study of the removal of pollutants from aqueous solutions [6,7]. It explains the second-order kinetics assuming that the solid surface has heterogeneous energy but does not suggest any mechanism for adsorption [7]. The Elovich model is represented as [8,9]
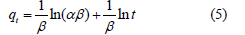
where α (mg/g.min) and β (g/mg) are constant. The parameter α (mg/g.min) is initial adsorption rate and β (mg/g.min) is the extent of surface coverage and activation energy for chemisorption.
Liquid film diffusion model: It is expressed as [10].
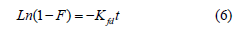
where Kfd (/min) is liquid film diffusion rate constant, and F=qt/ qe-
Modified freundlich equation: It was originally developed by Kuo and Lotse [5,11]

where k (L/g.min), Co (mg/L), t (min) and m are adsorption rate constant, initial concentration contact time and the Kuo-Lotse constant respectively. Its linear form is shown as:
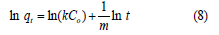
Bangham equation: Bangham equation is represented as [4,9].
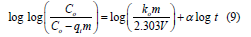
where m (g) is mass of the TRH (adsorbent) employed, V (mL) is volume of EBT dye solution, α (<1) and ko (mL/(g/L) are constants.
Adsorption isotherms
Adsorption isotherms including Langmuir, Freundlich, Dubinin- Radushkevich (D-R) and Temkin were employed to explore experimental data for adsorption of EBT from aqueous solution onto treated rice husk.
Langmuir isotherm: It is based on the maximum adsorption corresponds to the saturated monolayer of liquid molecules on the solid surface. It is given as follows [12].
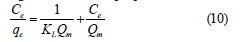
where KL (L/mg) is Langmuir constant and Qm (mg/g) is Langmuir monolayers adsorption capacity, Ce (mg/L) is supernatant concentration at equilibrium state of the system, and qe (mg/g) is the amount of dye adsorbed at equilibrium state of system. The essential characteristics of Langmuir isotherm can be shown in term of dimensionless constant separation factor RL that is given by [13].

The value of RL shows the shape of the isotherm to be either unfavorable (RL>1), linear (RL=1), favorable (0< RL>0), or irreversible (RL=0) [14].
Freundlich isotherm: It is an empirical relation utilized to explain the heterogeneous system. The Freundlich isotherm model is expressed as [15].
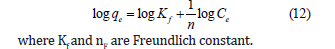
Dubinin radushkevich (D-R) isotherm: The Dubinin-Radushkevich (D-R) model is represented as [16].
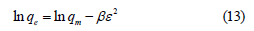
where β (mol2/KJ) is constant related to the adsorption energy, qm (mg/g) is maximum adsorption capacity and ε is the polanyi potential can be calculated by employing below equation.

where R is gas constant (8.31KJ/mol), Ce (mg/L) is supernatant concentration at equilibrium state and T (K) is absolute temperature. The mean free energy E (KJ/mol) can be determined by below relationship.

Temkin isotherm: The linear form of Temkin isotherm is represented as [16].
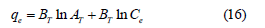
where BT=RT/bT, R is gas constant (8.31J/mol.K) and T (K) is absolute temperature. The constant bT is related to the heat of adsorption and AT is equilibrium binding constant coinciding to the maximum binding energy.
Thermodynamics study
For adsorption of EBT from an aqueous solution onto treated rice husk, the change in Gibb’s free energy (ΔGo), enthalpy (ΔHo) and entropy (ΔSo) were calculated to investigate adsorption thermodynamics as described [27,29,38].
The change in Gibb’s free energy (ΔGo), enthalpy (ΔHo) and entropy (ΔSo) were measured by using below relationship:
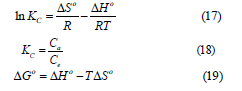
where Kc, ΔGo (KJ/mol), ΔHo (KJ/mol) and ΔSo (J/mol.K) are equilibrium constant, change in Gibb’s free energy, enthalpy and entropy respectively.
Results and Discussion
Point of zero charge and BET surface area
The pH of point of zero charge of adsorbent is very important factor in the adsorption process. pHpzc is the pH upon which charge on the adsorbent is zero and it is important to find out for any system. Determination of pHpzc of rice husk was done by the standard procedure as reported in our previous work [33]. Determined value of pHpzc is 5.84 [33]. Similarly BET surface area of treated rice husk was determined by using standard procedure as reported [33]. BET surface area for treated rice husk was found to be 1.03m2/g1.
SEM and FTIR
Morphology of untreated and NaOH treated rice husk was investigated by employing Scanning Electron Microscopy (SEM). Figure 3 represents morphology of untreated and NaOH treated rice husk. It was observed that the surface morphology of untreated rice husk was rough while morphology of NaOH treated rice husk was smooth. The structure of treated rice husk showed pores on it which were useful for adsorption of EBT from aqueous solution. FTIR spectrums of treated rice husk before and after adsorption of EBT are denoted in Figure 4. The peaks at 1217.0, 1365.4, 1737.8 and 1027.4cm-1 were associated to carboxyl group onto TRH in the range of reported peaks at 1208-1230, 1367-1371, 1740 and 1029cm-1 for carboxyl group [44,45]. The peak at 3400-3200cm-1 was attributed to the surface O-H stretching, while aliphatic C-H stretching had a broad band at 2921-2851cm-1. The peaks at 1737.8, 1435.6 and 1365. cm-1 was due to C=O stretching, OH bending of the adsorbed H2O and aliphatic C-H bending, respectively [46]. The absence of band related to non-conjugated carbonyl functional groups in the treated rice husk spectrum represents hydrolyses of carbonyl groups during NaOH treatment. Except, the band at 1074.0cm-1 coincided to anti-symmetric stretching vibration of Si-O, while at 476.2cm-1 represents bending vibration of Si-O-Si bond [44,47,48]. After adsorption of EBT onto Treated Rice Husk (TRH), the slight modifications in the bands of Treated Rice Husk (TRH) were observed as shown in Figure 4. The intensities of bands decreased after adsorption of EBT which suggests the involvement of these groups in adsorption of EBT onto Treated Rice Husk (TRH).
Figure 3:(a) SEM micrographs of untreated rice husk (RH), (b) SEM micrographs of NaOH treated rice husk (TRH).

Figure 4:FTIR Spectrum of TRH before and after adsorption of EBT dye.

Effect of operational factors on adsorptive removal of EBT and adsorption capacity
The effect of operating factors on the percentage removal of
EBT from an aqueous solution and adsorption capacity were investigated.
Its detail is given below:
Effect of contact time: Figure 5a represents the effect of contact
time on the percentage removal of EBT from aqueous solution
by Treated Rice Husk (TRH) and adsorption capacity at room
temperature. Results showed that the values of both percentage
removal of EBT and adsorption capacity were increased with
increasing contact time. It is similar to our previous work [27]. The
percentage removal of EBT and adsorption capacity was increased
from 35 to 81% and 0.71 to 1.61mg/g respectively with increasing
contact time. Table 2 provides an interesting comparison of EBT
removal from an aqueous solution by using different adsorbents.
The occurrence of fast adsorption of EBT in the beginning was
due to presence of large number of vacant adsorption sites onto
TRH. A large number of vacant adsorption sites were available for
interaction of Treated Rice Husk (TRH) with dye molecules. The
number of vacant active sites available onto TRH for adsorption
of EBT was decreased with progression of adsorption process.
It resulted to a slower increase in adsorption rate. The dynamic
equilibrium between adsorption and desorption of EBT was shown
by establishment of a plateau. Similar results were attained in our
previous work [49,50].
Figure 5:a) Effect of contact time, b) mass of treated rice husk (TRH), c) initial concentration of EBT

Table 2:The percentage of removal of EBT from aqueous solution by using different adsorbents.

Effect of mass of treated rice husk (TRH): Figure 5b shows the influence of mass of Treated Rice Husk (TRH) onto the percentage removal of EBT from an aqueous solution and adsorption capacity at room temperature. It was noted that the percentage removal of EBT from an aqueous solution was increased from 62 to 71% due to increase in active sites for adsorption process with increasing mass of treated rice husk [27]. On the contrary, adsorption capacity was decreased from 6.17 to 1.61mg/g with increasing mass of TRH. The decrease in adsorption capacity with increase in mass of treated rice husk from 0.04 to 0.20g was associated to limited initial concentration of EBT into aqueous solution [27,51]. Herein, the determined optimum mass of treated rice husk was found to be 0.20g which was employed for further research.
Effect of initial concentration: Figure 5c depicts the effect of initial concentration on the percentage removal of EBT from an aqueous solution by using Treated Rice Husk (TRH) and adsorption capacity at room temperature. It was noted that the percentage removal of EBT was deceased from 71 to 42% with increasing concentration of EBT aqueous solution at room temperature because the active sites of Treated Rice Husk (TRH) became saturated by enhancing the initial concentration of EBT aqueous solution from 20 to 400mg/L which leads to decrease in the adsorptive removal of dye [27]. Contrary, adsorption capacity was found to be enhanced from 1.61 to 17.0mg/g with increasing initial concentration of EBT aqueous solution from 20 to 400mg/L at room temperature. It was attributed to the higher initial concentration of EBT aqueous solution, which enhanced the transportation of its molecules from solution to Treated Rice Husk (TRH) surface. It results in the higher interaction between EBT molecules and the Treated Rice Husk (TRH) surface. From this we concluded that the initial concentration of dye had a positive effect on adsorption capacity of Treated Rice Husk (TRH) when initial concentration of dye was low there may be some active sites on adsorbent which remain vacant due to low dye concentration but as dye concentration was increased there are enough dye molecules to be adsorbed on active sites leaving least chances for active sites to remain vacant or un-adsorbed.
Effect of temperature: The influence of temperature on the percentage removal of EBT from aqueous solution by using TRH and adsorption capacity is shown in Figure 6a. From here, it was observed that both the percentage removal of EBT and adsorption capacity was enhanced with rise in temperature. The percentage removal of EBT was found to be increased from 71 to 84% and adsorption capacity from 1.41 to 1.68mg/g with rise in temperature from 298 to 333K. From here it was concluded that adsorption of EBT onto TRH from aqueous solution was an endothermic process.
Figure 6:a) Effect of temperature, b) effect of pH on the percentage removal of EBT from aqueous solution and adsorption capacity.

Figure 7:Mechanism of EBT adsorption onto TRH from an aqueous solution.

Effect of pH: It is crucial to investigate pH influence on adsorption of EBT onto treated rice husk because adsorption process is deeply influenced by the acidity of the aqueous solution. Figure 6b represents the effect of pH on EBT removal from an aqueous solution and adsorption capacity. It is noted that both EBT percentage removal and adsorption capacity were increased till pH 4, a minute decrease was observed till pH 7 and at pH 8 onwards a decreasing trend was noticed. It is associated with the surface charge as well as the degree of ionization and speciation of different pollutants studied. In acid pH range, adsorption of EBT was higher which is probably due to the presence of H+ ions that can interact with anionic groups of the dye and result in electrostatic force of interactions between negatively charges surface of treated rice husk and positively charged dye molecules [52,53-55]. In addition, the mechanism of EBT adsorption onto TRH is represented in Figure 7.
Adsorption kinetics study
Figure 8a represents the plot of pseudo-first-order model for adsorption of EBT from aqueous solution onto Treated Rice Husk (TRH) and the calculated values of its parameters (k1 & qe) are given in Table 3. It can be seen that there was a difference between calculated adsorption capacity (qe,cal= 1.481mg/g) and experimental adsorption capacity (qe, exp= 1.616mg/g). Besides, the value o correlation coefficient (R2) for pseudo-first-order model was 0.975 which shows nonlinearity of experimental data. Hence, the pseudo- first-order model does not explain the rate process. The plot of pseudo-second-order model for adsorption of EBT onto TRH is shown in Figure 8b and the measured values of its parameters are given in Table 3. From here, it was observed that the calculated value of adsorption capacity (1.869mg/g) was close to experimental value (1.616mg/g). Moreover, the value of correlation coefficient (R2) was close to unity (R2>0.996) explaining linear behavior of experimental data. From this we concluded that experimental data for adsorption of EBT from an aqueous solution onto Treated Rice Husk (TRH) obeyed pseudo-second-order model. Similarly, the plot of Elovich model for adsorption of EBT from an aqueous solution onto TRH is shown in Figure 8c. The value of correlation coefficient (R2) was 0.881. The values of Elovich coefficients could be measured from the plot’s qt versus lnt. The measured values of α and β are given in Table 3. The applicability of the simple Elovich equation for present experimental data is generally in agreement with other researcher’s results that the Elovich equation was able to describe properly the kinetics of EBT adsorption onto Treated Rice Husk (TRH) [56].
Figure 8:(a) Pseudo-first-order model, (b) pseudo-second-order model, (c) Elovich model for adsorption of EBT from aqueous solution onto treated rice husk (TRH).

Table 3:The calculated values of adsorption kinetics parameters for adsorption of EBT from aqueous solution onto Treated Rice Husk (TRH).

(qe: mg/g; k1: (/min); k2: g/mg.min; α: mg/g.min; β: g/mg; kfd: (/min); k: L/g.min; ko: mL/g/L)
Figure 9a shows the plot of liquid film diffusion model for adsorption of EBT from an aqueous solution onto Treated Rice Husk (TRH). The calculated value of Kfd from slope of the linear plot is given in Table 3. It has been observed that the points were not much dispersed and the value of correlation coefficient (R2) was 0.975. Hence, the liquid film diffusion model was satisfactory to explain experimental data for adsorption of EBT from aqueous solution onto TRH. The plot of modified Freundlich equation for adsorption of EBT from aqueous solution onto TRH is depicted in Figure 9b and the calculated values of m and k are given in Table 3. From here, it was observed that the value of correlation coefficient (R2=0.954) was lower than pseudo-second-order model. Therefore, modified Freundlich equation can’t explain experiment data for adsorption of EBT from aqueous solution onto TRH. Figure 9c indicates the plot of Bangham equation for adsorption of EBT onto TRH and the measured values of its parameters are given in Table 3. The value of correlation coefficient (R2=0.954) was smaller than pseudo-second-order model. For adsorption of EBT from aqueous solution onto TRH, the double logarithmic plot did not give linear curve indicating that the diffusion of adsorbate (EBT) into pores of the adsorbent (TRH) is not the only rate controlling step [34]. It may be that both film and pore diffusion were crucial to different extent in adsorption of EBT from aqueous solution onto Treated Rice Husk (TRH).
Figure 9:(a) Liquid film diffusion model, (b) modified Freundlich equation, (c) Bangham equation for adsorption of EBT onto TRH from an aqueous solution.

Adsorption isotherms
Adsorption isotherms including Langmuir, Freundlich, Dubinin- Radushkevich (D-R) and Temkin were applied to experimental data for adsorption of EBT from an aqueous solution onto TRH. Figure 10a represents Langmuir isotherm for adsorption of EBT from an aqueous solution onto TRH and the calculated values of its parameters are given in Table 4. The value of correlation coefficient (R2= 0.941) showed that experimental data for adsorption of EBT onto TRH fitted to Langmuir isotherm. The measured value of RL (0.19-0.82) represents that adsorption of EBT from aqueous solution onto TRH was favorable process. Figure 10b represents Freundlich isotherm for adsorption of EBT and the attained values of its parameters is given in Table 4. The value of correlation coefficient (R2=0.962) exhibited that experiment data fitted to Freundlich isotherm. The value of Freundlich constant ‘n’ ranges from 2-10 representing good adsorption, 1-2 moderate adsorption and less than one shows poor adsorption [32,33]. By comparison of Freundlich and Langmuir isotherm data from regression values, it can be found out that Freundlich isotherm fits well to experimental data as compared to Langmuir isotherm. Similarly, Figure 10c shows the D-R isotherm for adsorption of EBT onto TRH. The values of D-R constant (Cm) and β were calculated from intercept and slope. These attained values are given in Table 4. The measured value of mean adsorption energy for adsorption of EBT onto TRH was 0.609KJ/ mol representing that adsorption of EBT onto TRH was physical adsorption process [23,24]. Moreover, the plot of Temkin isotherm for adsorption of EBT onto TRH is shown in Figure 10d. The value of correlation coefficient (R2=0.891) was lower than Freundlich isotherm representing that experimental data is not fitted to Temkin isotherm. The measured values of bT and AT are given Table 4.
Figure 10:Langmuir isotherm, (b) Freundlich isotherm, (c) Dubinin-Radushkevich (D-R) isotherm, (d) Temkin isotherm for adsorption of EBT from aqueous solution onto treated rice husk (TRH).

Table 4:Determined parameters of adsorption isotherms for adsorption of EBT from aqueous solution onto Treated Rice Husk (TRH).

(Qm (mol/g), KL (L/mol), kF ((mg/g) (L/mg)1/n), Cm (mol/g), β (mol2/J2), E (kJ/mol))
Adsorption thermodynamics study
The plot of 1/T verses lnKc for adsorption of EBT from aqueous solution onto Treated Rice Husk (TRH) is represented in Figure 11 and the determined values of thermodynamic parameters (ΔG°, ΔS° & ΔH°) are given in Table 5. The negative value of Gibb’s free energy (ΔG°) suggested that adsorption of EBT from aqueous solution onto Treated Rice Husk (TRH) was spontaneous in nature. With rise in temperature from 298 to 333K, the decline in the values of Gibb’s free energy indicated the decrease in feasibility of adsorption process at elevated temperature. The positive value of entropy (ΔSo= 18.26J/K.mol) denoted the increase in randomness at the TRH-EBT interface during adsorption of EBT from aqueous solution onto Treated Rice Husk (TRH). In addition, the positive value of enthalpy (ΔHo= 20.57KJ/mol) demonstrated that adsorption of EBT from aqueous solution onto Treated Rice Husk (TRH) was an endothermic process.
Figure 11:Plot of 1/T vs lnKc for adsorption of EBT from aqueous solution onto treated rice husk (TRH).

Table 5:Calculated thermodynamic parameters for adsorption of EBT from aqueous solution onto Treated Rice Husk (TRH).

Regeneration studies
Cost effectiveness can be considered as prime criterion while designing the adsorbent meant for adsorption process and it can be easily achieved by regenerating the material for a number of cycles. The major challenge still remains the efficiency of the adsorbent in each cycle. In the current work, the ability of TRH to be recycled was studied for 5 consecutive cycles. There was no appreciable loss in the percentage removal of dye for the first three consecutive cycles, however a minute decrease was observed in the fourth cycle which became pronounced in the 5th one. This typical behavior generally occurs due to the strong retention of some molecules of dyes on the surface of adsorbent with the passage of time. These molecules start blocking the active sites and thus result in gradual loss of adsorbent’s efficiency over a number of cycles. Figure 12 shows the regeneration behavior of adsorbent in terms of percentage removal.
Figure 12:Regeneration behavior of adsorbent in terms of percentage removal.

Conclusion
In this article, adsorption of EBT from an aqueous solution onto Treated Rice Husk (TRH) was investigated in detail at room temperature This study revealed that abundantly available agricultural waste treated rice husk was a cost-effective adsorbent for decontamination of EBT. FTIR spectroscopy confirmed adsorption of EBT onto Treated Rice Husk (TRH). The percentage removal of EBT was increased with increase in contact time, mass of TRH and temperature while decreased with initial concentration. Contrary, adsorption capacity was increased with contact time, initial concentration of EBT aqueous solution and temperature while deceased with mass of Treated Rice Husk (TRH). A kinetics study showed that adsorption of EBT onto Treated Rice Husk (TRH) fitted well to pseudo-second-order kinetics. The adsorption equilibrium study concluded that Freundlich isotherm fitted well with regression value of 0.962. Results of thermodynamic study suggested that adsorption of EBT onto Treated Rice Husk (TRH) was an endothermic process and negative value of change in Gibbs free energy confirmed feasibility of EBT adsorption process. On the basis of this study, it can be concluded that abundantly and locally available inexpensive rice husk has great potential to be utilized for the removal of EBT from bulk aqueous solutions for the safe disposal of industrial as well as textile effluents and will provide an alternative solution to environmental damages caused by these dyes.
References
- Karnjkar YS, Dinde RM, Dinde NM, Bawankar KN, Hinge SP, et al. (2015) Degradation of magenta dye using different approaches based on ultrasonic and ultraviolet irradiations: Comparison of effectiveness and effect of additives for intensification. Ultrasonics Sonochemistry 27: 117-124.
- Tripathi A, Ranjan MR (2015) Heavy metal removal from wastewater using low-cost adsorbents. Journal of Bioremediation & Biodegradation 6(6): 6-12.
- Ahmad T, Belwal T, Li L, Ramola S, Aadil RM, et al. (2020) Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends in Food Science & Technology 99: 21-33.
- Peng Q, Liu M, Zheng J, Zhou C (2015) Adsorption of dyes in aqueous solutions by chitosan-halloysite nanotubes composite hydrogel beads. Microporous and Mesoporous Materials 201: 190-201.
- Tan KB, Vakili M, Horri BA, Poh PE, Abdullah AZ, et al. (2015) Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Separation and Purification Technology 150: 229-242.
- Liu L, Gao ZY, Su XP, Chen X, Jiang L, et al. (2015) Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent. ACS Sustain Chem Eng 3: 432-442.
- Bansal M, Patnala PK, Dugmore T (2020) Adsorption of Eriochrome Black-T(EBT) using tea waste as a low cost adsorbent by batch studies: A green approach for dye effluent treatments. Current Research in Green and Sustainable Chemistry 3: 100036.
- Sharifpour E, Khafri HZ, Ghaedi M, Asfaram A, Jannesar R (2018) Isotherms and kinetic study of ultrasound-assisted adsorption of malachite green and Pb2+ ions from aqueous samples by copper sulfide nanorods loaded on activated carbon: Experimental design optimization. Ultrasonics Sonochemistry 40: 373-382.
- Lozano MM, Escamilla VM, García ER, Medina RL, Romero GR, et al. (2017) Sonophotocatalytic degradation of orange II dye using low cost photocatalyst. Journal of Cleaner Production 148: 836-844.
- Li G, Liu B, Bai L, Shi Z, Tang X, et al. (2020) Improving the performance of loose nanofiltration membranes by poly-dopamine/zwitterionic polymer coating with hydroxyl radical activation. Separation and Purification Technology 238: 116412.
- Yu J, Zhang D, Ren W, Liu B (2019) Transport of enterococcus faecalis in granular activated carbon column: Potential energy, migration and release. Colloids and Surfaces B: Biointerfaces183: 110415.
- Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions: A review. J Hazard Mater 167(1-3): 1-9.
- Ghoreishi SM, Haghighi R (2003) Chemical catalytic reaction and biological oxidation for treatment of non-biodegradable textile effluent. Chemical Engineering Journal 95(1-3): 163-169.
- Yu J, Zou A, He W, Liu B (2020) Adsorption of mixed dye system with cetyltrimethylammonium bromide modified sepiolite: Characterization, performance, kinetics and thermodynamics. Water 12: 981.
- Shao S, Fu W, Li X, Shi D, Jiang Y, et al. (2019) Membrane fouling by the aggregations formed from oppositely charged organic foulants. Water Res 159: 95-101.
- Garg VK, Gupta R, Yadav AB, Kumar R (2003) Dye removal from aqueous solution by adsorption on treated sawdust. Bioresour Technol 89(2): 121-124.
- Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J Hazard Mater 97(1-3): 219-243.
- Ali I, Asim M, Khan TA (2012) Low-cost adsorbents for the removal of organic pollutants from waste water. Journal of Environmental Management 113: 170-183.
- Ihsanullah, Abbas A, Al-Amer AM, Laoui T, Al-Marri MJ, et al. (2016) Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical Review of Adsorption Applications. Separation and Purification Technology 157: 141-161.
- Tillotson MJ, Brett PM, Bennett RA, Crespo RG (2015) Adsorption of organic molecules at the TiO2(110) surface: The effect of van der waals interactions. Surface Science 632: 142-153.
- Song J, Xu T, Gordin ML, Zhu P, Lv D, et al. (2014) Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium-sulfur batteries. Advanced Functional Materials 24(9): 1243-1250.
- Grassi M, Rizzo L, Farina A (2013) Endocrine disruptors compounds, pharmaceuticals and personal care products in urban wastewater: Implications for agricultural reuse and their removal by adsorption process. Environ Sci Poll Res 20(6): 3616-3628.
- Khan MI, Zafar S, Khan MA, Mumtaz F, Prapamonthon P, et al. (2018) Bougainvillea glabra leaves for adsorption of congo red from wastewater. Fresenius Environmental Bulletin 27: 1456-1465.
- Khan MI, Zafar S, Buzdar AR, Azhar MF, Hassan W, et al. (2018) Use of citrus sinensis leaves as a bioadsorbent for removal of congo red dye from aqueous solution. Fresenius Environmental Bulletin 27: 4679-4688.
- Khan MI, Zafar S, Azhar MF, Buzdar AR, Hassan W, et al. (2018) Leaves powder of syzgium cumini as an adsorbent for removal of congo red dye from aqueous solution Fresenius Environmental Bulletin 27: 3342-3350.
- Khan MI, Zafar S, Ahmad HB, Hussain M, Shafiq Z (2015) Use of morus albal leaves as bioadsorbent for the removal of congo red dye. Fresenius Environmental Bulletin 24(6a): 2251-2258.
- Khan MI, Shanableh A, Fernandez J, Lashari MH, Shahida S, et al. (2021) Synthesis of DMEA-grafted anion exchange membrane for adsorptive discharge of methyl orange from wastewaters. Membranes 11(3): 166.
- Khan MI, Ansari TM, Zafar S, Buzdar AR, Khan MA, et al. (2018) Acid green-25 removal from wastewater by anion exchange membrane: Adsorption kinetic and thermodynamic studies. Membrane Water Treat 9(2): 79-85.
- Khan MA, Khan MI, Zafar S (2017) Removal of different anionic dyes from aqueous solution by anion exchange membrane. Membrane Water Treat 8(3): 259-277.
- Khan MI, Wu L, Mondal AN, Yao Z, Ge L, et al. (2016) Adsorption of methyl orange from aqueous solution on anion exchange membranes: Adsorption kinetics and equilibrium. Membrane Water Treat 7(1): 23-38.
- Zafar S, Khan MI, Khraisheh M, Shahida S, Khalid N, et al. (2018) Effective removal of lanthanum ions from aqueous solution using Rice husk: Impact of experimental variables. Desalination and Water Treat 132: 263-273.
- Zafar S, Khan MI, Khraisheh M, Shahida S, Javed T, et al. (2019) Use of rice husk as an effective sorbent for the removal of cerium ions from aqueous solution: Kinetic, equilibrium and thermodynamic studies. Desalination and Water Treat 150: 124-135.
- Zafar S, Khan MI, Khraisheh M, Lashari MH, Shahida S, et al. (2019) Kinetic, equilibrium and thermodynamic studies for adsorption of nickel ions onto husk of oryza sativa. Desalination and Water Treat 167: 277-290.
- Zafar S, Khan MI, Rehman H, Garcia JF, Shahida S, et al. (2020) Kinetic, equilibrium and thermodynamic studies for adsorptive removal of cobalt ions by rice husk from aqueous solution. Desalination and Water Treat 204: 285-296.
- O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour Technol 99(15): 6709-6724.
- Zafar S, Khan MI, Lashari MH, Khraisheh M, Almomani F, et al. (2020) Removal of copper ions from aqueous solution using NaOH-treated rice husk. Emergent Materials 3: 857-870.
- Khan MI, Su J, Guo L (2021) Development of triethanolamine functionalized-anion exchange membrane for adsorptive removal of methyl orange from aqueous solution. Desalination and Water Treat 209: 342-352.
- Khan MI, Akhtar S, Zafar S, Shaheen A, Khan MA, et al. (2015) Removal of congo red from aqueous solution by anion exchange membrane (EBTAC): Adsorption kinetics and thermodynamics. Materials 8(7): 4147-4161.
- Khan MI, Shanaleh A, Elboughdiri N, Manzoor S, Mubeen S, et al. (2022) Application of NaOH modified rice husk as a potential sorbent for removal of Congo red from an aqueous solution. Desalination and Water Treat 273: 221-235.
- Ayyaril SS, Khan MI, Shanableh A, Bhattacharjee S, Oliveira DM (2022) Fabrication of nano-activated charcoal incorporated sodium alginate-based cross-linked membrane for rhodamine B adsorption from an aqueous solution. Desalination and Water Treat 278: 239-250.
- Zafar S, Khan MI, Elboughdiri N, Lashari MH, Shanableh A, et al. (2022) Adsorption performance of rice husk towards copper ions from wastewater. Desalination and Water Treat 258: 133-142.
- Almanassra IW, Khan MI, Atieh MA, Shanableh A (2022) Adsorption of lead ions from an aqueous solution onto NaOH-modified rice husk. Desalination and Water Treat 262: 152-167.
- Almanassra IW, Khan MI, Chatla A, Atieh MA, Shanableh A (2022) Utilization of palm leaves as an extraordinary adsorbent for the removal of Pb (II) from an aqueous solution. Desalination and Water Treat 271: 206-219.
- Srivastaa VC, Mall ID, Mishra IM (2006) Characterization of mesoporous Rice Husk Ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J Hazard Mater 134(1-3): 257-267.
- Kazy SK, Sar P, Sen AK, Singh SP, D'Souza SF (2002) Extracellular polysaccha-rides of a copper sensitive and a copper resistant pseudomonas aeruginosa stain: Synthesis, chemical nature and copper binding. World Journal of Microbiology and Biotechnology 18(6): 583-588.
- Kazy SK, D'Souza SF, Sar P (2009) Uranium and thorium sequestration by a Pseudomonas sp.: Mechanism and chemical characterization. J Hazard Mater 163(1): 65-72.
- Mei XY, Ji Q, Mei WD, Ying CH, Jun G, et al. (2010) Preparation of amorphous silica from oil shale residue and surface modification by silane coupling agent. Oil Shale 27: 37-46.
- Ludueña L, Fasce D, Alvarez VA, Stefani PM (2011) Nanocellulose from rice husk following alkaline treatment to remove silica. BioResour 6(2): 1440-1453.
- Khan MI, Lashari MH, Khraisheh M, Shahida S, Zafar S, et al. (2019) Adsorption kinetic, equilibrium and thermodynamic studies of Eosin-B onto anion exchange membrane. Desalination and Water Treat 155: 84-93.
- Khan MI, Shanableh A, Nasir N, Shahida S (2021) Adsorptive removal of methyl orange from wastewaters by the commercial anion exchange membrane EPTAC. Desalination and Water Treat 234: 245-254.
- Ho YS (2006) Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res 40(1): 119-125.
- Zubair M, Jarrah N, Manzar MS, Al-Harthi M, Daud M, et al. (2017) Adsorption of eriochrome black T from aqueous phase on MgAl-, CoAl- and NiFe- calcined layered double hydroxides: Kinetic, equilibrium and thermodynamic studies. Journal of Molecular Liquids 230: 344-352.
- Kaur Y, Jasrotia T, Kumar R, Chaudhary GR, Chaudhary S (2021) Adsorptive removal of eriochrome black T (EBT) dye by using surface-active low-cost zinc oxide nanoparticles: A comparative overview. Chemosphere 278: 130366.
- Zubair M, Aziz HA, Ahmad MA, Ihsanullah I, Al-Harthi MA (2021) Adsorption and reusability performance of M-Fe (M = Co, Cu, Zn and Ni) layered double hydroxides for the removal of hazardous eriochrome black T dye from different water streams. Journal of Water Process Engineering 42: 102060.
- Muazu ND, Jarrah N, Kazeem TS, Zubair M, Al-Harthi M (2018) Bentonite-layered double hydroxide composite for enhanced aqueous adsorption of eriochrome black T. Applied Clay Science 161: 23-34.
- Chien SH, Clayton WR (1980) Application of elovich equation to the kinetics of phosphate release and sorption in soils. Soil Science Society of America Journal 44(2): 265-268.
© 2024 Muhammad Imran Khan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






