- Submissions

Full Text
Progress in Petrochemical Science
4,4-Dimethoxy-2-butanone as 1,3-Dielectrophylic 3-Carbon Building Block: New Route for the Synthesis of Toluene, o-Xylene, Naphthalenes and Pyrimidines
Charanraj TP1, Ramachandra P2, Ramesh N3 and Junjappa H1*
1 Chemistry Research Unit, REVA Institute of Science and Management, India
2 Department of Chemistry, REVA Institute f Technology and Management, India
3 Director Planning, REVA University, India
*Corresponding author: Junjappa H, Chemistry Research Unit, REVA Institute of Science & Management, Bangalore, India
Submission: July 12, 2018;Published: August 24, 2018

ISSN 2637-8035Volume2 Issue4
Abstract
4,4-dimethoxy-2-butanone 1 is shown to react with allyl magnesium chloride/allyl bromide in the presence of zinc to yield the corresponding toluene in 63% and 52% yields respectively. Similarly, crotyl bromide reacted with 1 in the presence of zinc to yield o-xylene in 32% yield. 1 was also reacted with benzyl magnesium chloride and phenyl acetonitrile to yield the corresponding naphthalenes in 53% and 61% yields respectively. The ketone 1 was reacted with guanidines to yield the corresponding pyrimidines in 58% and 66% yields respectively.
Keywords: 4,4-dimethoxy-2-butanone; Zinc; Toluene; Naphthalene; Pyrimidine
Introduction
figure 1:
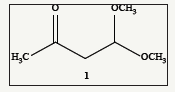
The 4, 4-Dimethoxy-2-butanone 1 (Figure 1) is an interesting three carbon 1, 3-dielectrophilic building block. Which is commercially available and a few of its synthetic applications have been reported in recent years. One of the earliest reactions [1] of 1 is reported to have reacted with hydrazine hydrate to yield the corresponding 3-methylpyrazole in excellent yield. Recently there are a number of patents [2-5] described on the reaction of various phenyl hydrazines with 1 to obtain the corresponding aryl methyl pyrazoles in around 60-68% overall yields. In one of their publications Nielsen et al. [6] have reacted p-methyl benzyl magnesium chloride with 1 to give initially the corresponding carbinol acetal in good yields which on treatment with hydrobromic acid in acetic acid, the corresponding 3, 7-dimethyl naphthalene was formed in 24% yield. Similarly, Butter field and coworkers [7] as a part of their total synthesis of corannulene reacted m-methyl benzyl magnesium chloride with 1, initially to afford the corresponding carbinol which on treatment with acetic acid and 0.8M sulfuric acid at room temperature the corresponding 2, 7-dimethyl naphthalene was formed in 71% yield. Since many years we have been working on α-oxoketenedithioacetal chemistry [8-18] as a useful class of 1, 3-dielectrophylic building blocks for the synthesis of a variety of 5 and 6 membered heterocycles and aromatics. We wanted to extend these ideas to use 4, 4-dimethoxy butanone as a useful 1, 3-dielectrophilic building block and some of these selected reactions are reported in this communication.
Results and Discussion
To begin with the allyl magnesium bromide [19] 2 was reacted with 1 at room temperature to afford the corresponding carbinol acetal 4 in 90% yields. The acetal was then refluxed with boron trifluoride etherate to afford the corresponding toluene 5 in 63% yield (Scheme 1). Thus, allyl bromide 3 was reacted with 1 in the presence of zinc [20] and saturated ammonium chloride when the carbinol acetal 4 was obtained in 70% yield. The acetal was then refluxed with boron trifluoride etherate as described above to afford the expected toluene 5 (Scheme 1) in 52% yield. The structure of toluene was fully confirmed by its super imposable IR spectra and other analytical data
Figure :
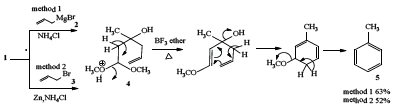
Scheme 1: Synthesis of toluene from method 1 and method 2.
We next extended this reaction for the synthesis of p-xylene (Scheme 2) by reacting crotyl zinc bromide [21] and it was reasoned that the organo zinc bromide prepared from crotyl bromide would react with 1b in 1,2 addition mode to yield the corresponding carbinol acetal 8, which on acid assisted cyclization would give p-xylene 9 (path a). However, the anion reacted from its third carbon with 1b to yield the different carbinol acetal 10 which on cyclization the corresponding o-xylene 11 (path b) was obtained in 32% yield (Scheme 2).
Figure :
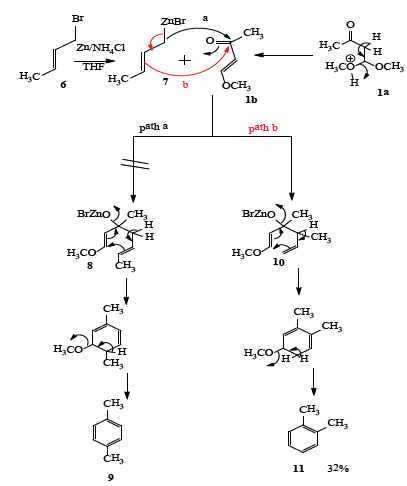
Scheme 2: Synthesis of o-xylene
Figure :
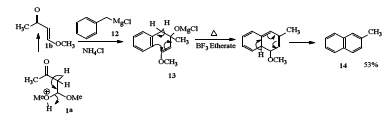
In the next experiment, benzyl magnesium chloride [22] 12 was reacted with 1 to afford the corresponding carbinol acetal 13 in high yield. It was then cyclized in the presence of boron trifluoride etherate, the corresponding 2-methyl naphthalene 14 (Scheme 3) was obtained in 53% yield.
Figure :
Scheme 3: Synthesis of 2-Methylnaphthalene.
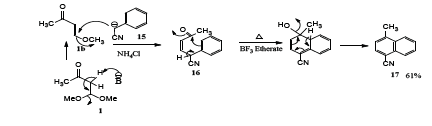
Also, phenyl acetonitrile [23] 15 was reacted with 1 in the presence of sodium hydride (Scheme 4) using a mixture of dimethylformamide and tetrahydrofuran (1:4) to afford the corresponding 1,4-addition-elimination product 16 which on refluxing with boron trifluoride etherate, the corresponding 1-methyl-4-cyanonaphthalene 17 was obtained in 61% yield (Scheme 4)
Figure :
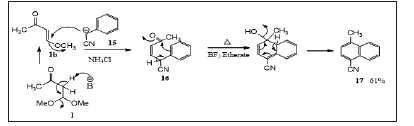
Scheme 4: Synthesis of 1-Methyl-4-cyanonaphthalene.
The building block 1 was also reacted with guanidine nitrate [24-28] in the presence of sodium ethoxide in boiling ethanol to give the corresponding 2-amino-4-methylpyrimidine 18 in 66% yield (Scheme 5).
Figure :
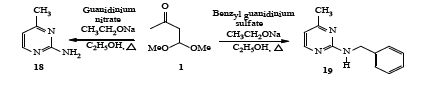
Scheme 5: Synthesis of 2-Amino-4-methylpyrimidine and 2-Benzylamino-4-methylpyrimidine.
Similarly, when benzyl guanidine [24-28] was reacted with 1 as described above to afford the corresponding 2-benzylamino-4- methylpyrimidine 19 in 58% yield (Scheme 5).
Experimental
General instructions
All the reagents and solvents used were purchased from commercial suppliers (Aldrich, TCI and SD fine chemicals) and used without further purification. 1H and 13C NMR spectra were recorded on 400MHz and 500MHz spectrophotometer using CDCl3 as the solvent. Chemical shift values are reported in ppm taking tetramethylsilane as the internal standard, and J values are given in hertz. HRMS data were acquired on a time of flight (TOF) mass spectrometer (Waters Q-TOF Premier-HAB213). IR spectra of all the compounds were obtained by PerkinElmer Spectrum version 10.3.06 IR spectrometer. Reactions were monitored by thin layer chromatography (TLC) using precoated silica gel plates, visualized by UV light. Melting points were determined using Thiele tube apparatus and are uncorrected.
Experimental Procedure
Procedure for the synthesis of Toluene 5.
Method 1: Synthesis of Toluene from allyl magnesium bromide: In a neat reaction under
argon, allyl magnesium bromide 2 (22.7ml) was slowly added to 1 (3g, 22.7mmol) at 0 °C and the reaction mixture was stirred at that temperature for 45min. (TLC). The reaction mixture was treated with saturated ammonium chloride solution (10ml) followed by extraction with ether which was dried, and the solvent was removed under reduced pressure to afford the corresponding carbinol 4 (3.53g, 20.28mmol) in 90% yield. The carbinol acetal was then refluxed with boron trifluoride etherate (6.18g, 50.65mmol) for 30minutes (TLC). The reaction mixture was poured into saturated NaHCO3 solution and extracted with ether (30ml), washed (H2O), dried (Na2SO4) and evaporated to yield the crude toluene 5 which was purified through vacuum distillation to give pure toluene in 63% yield; [Found: 1H NMR (400MHz CDC13) 2.34 (3H, s, CH3), 7.25-7.14 (5H, m, ArH); 13C NMR (99.5MHz, CDCl3) 21.5, 125.4, 128.3, 129.1, 138.0; HRMS: MH+, found 93.0703. C7H9 requires 93.0704.
Method 2: Synthesis of Toluene from allyl bromide: To the mixture of butanone 1 (3 g, 22.7mmol), allyl bromide (5.5g, 45.45mmol), Zn powder (2.97g, 45.45mmol) and THF (3ml), saturated NH4Cl solution (4ml) was added. The reaction mixture underwent exothermic reaction for 20 minutes to give carbinol 4 (2.76g, 15.9mmol) in 70% yield. The carbinol acetal was then refluxed with boron trifluoride etherate (4.83g, 39. mmol) for 30minutes. The reaction mixture was poured into saturated NaHCO3 solution and extracted with ether (30ml), washed (H2O), dried (Na2SO4) and evaporated to yield the crude toluene 5 which was purified through vacuum distillation to afford the desired toluene in 52% yield. The structure was confirmed by super imposable IR spectra. [Found: 1H NMR (400 MHz CDC13) 2.34 (3H, s, CH3), 7.25- 7.14 (5H, m, ArH); 13C NMR (99.5 MHz, CDCl3) 21.5, 125.4, 128.3, 129.1, 138.0; HRMS: MH+, found 93.0703. C7H9 requires 93.0704.
Procedure for the synthesis of o-Xylene 11: To the mixture of butanone 1 (4g, 30mmol), crotyl bromide (8g, 60mmol), Zn powder (3.92g, 60mmol) and THF (3ml), saturated NH4Cl solution (4ml) was added. The reaction mixture underwent exothermic reaction for 20 minutes to give carbinol 10 (2.84g, 15mmol) in 48 % yield. The carbinol acetal was then refluxed with boron trifluoride etherate (5.36g, 37.7mmol) for 30minutes. The reaction mixture was poured into saturated NaHCO3 solution and extracted with ether (30ml), washed (H2O), dried (Na2SO4) and evaporated to yield the crude o-xylene which was purified through controlled vacuum distillation to afford the desired o-xylene 11 in 32 % yield. The structure was confirmed by super imposable IR spectra; [Found: 1H NMR (400 MHz CDC13) 2.39 (6H, s, CH3), 7.26-7.23 (4H, m, ArH); 13C NMR (99.5 MHz, CDCl3) 19.8, 125.9, 129.7, 136.5.
Procedure for the synthesis of 2-Methylnaphthalene14: In a neat reaction under argon, benzyl magnesium bromide (22.7ml) 12 was slowly added to 1 (3g, 22.7 mmol) at 0 °C and the reaction mixture was stirred at that temperature for 45min. The reaction mixture was treated with saturated ammonium chloride solution (10ml) followed by extraction with ether which was dried and the solvent was removed under reduced pressure to afford the corresponding carbinol acetal (4.58g, 20.45mmol) 13 in 90% yield. The carbinol acetal was then refluxed with boron trifluoride etherate (6.23g, 51.1mmol) for 30minutes. The reaction mixture was poured into saturated NaHCO3 solution and extracted with ether (30ml), washed (H2O), dried (Na2SO4) and evaporated to yield the crude naphthalene which was purified through silica gel column eluting with petroleum ether to afford the desired 2-methylnaphthalene 14 in 53% yield; [Found: IR (solid film) 3051, 3014, 2918, 2860, 1913, 1633, 1508, 810cm-1; 1H NMR (400 MHz CDC13) 2.47 (3H, s, CH3), 7.27 (1H, d, J=8.4 Hz, ArH), 7.43-7.35 (2H, m, ArH), 7.57 (1H, s, ArH), 7.77-7.69 (3H, m, ArH) ; 13C NMR (125.76 MHz, CDCl3) 21.7, 125.0, 125.9, 126.9, 127.3, 127.6, 127.7, 128.1, 131.7, 133.7, 135.4.
Procedure for the synthesis of 2-Methyl-4- cyanonaphthalene 17: To a well stirred suspension of sodium hydride (0.60g, 25.25mmol, 60% purity) in DMF (5ml) and toluene (10ml) at 0 °C, a solution of benzyl cyanide 15 (1.15g, 9.84mmol) in toluene (5ml) was added drop wise and continued stirring for 15minutes. The butanone 1 (1g, 7.57mmol) in toluene (5ml) was then added slowly and the reaction mixture was stirred at room temperature for 6h (TLC). It was then poured into saturated ammonium chloride solution and extracted with ethyl acetate (30X2). The combined organic extracts was washed with water, dried (Na2SO4) and evaporated to yield crude 1, 4-additionelimination adduct 16. This adduct was treated with borontrifluoro etherate (2.08g, 17mmol) and refluxed for 30min (TLC). The reaction mixture was then poured into saturated solution of sodium bicarbonate (20ml) and extracted with ethyl acetate, washed (H2O) ,dried (Na2SO4) and the solvent was removed to give the corresponding 2-methyl-4-cyanonaphthalene 17 which was passed through silica gel column eluting with petroleum ether and ethyl acetate (96:4) to afford colorless liquid in 61% yield.; [Found: IR (solid film) 3406, 3041, 2927, 2219, 1719, 1163, 758cm-1; 1H NMR (400 MHz CDC13) 2.65 (3H, s, SCH3), 7.52 (1H, t, J=8.0 Hz, ArH), 7.62 (1H, t, J=7.2 Hz, ArH), 7.98 (1H, d, J=8.0 Hz, ArH), 8.13 (1H, d, J=8.4 Hz, ArH) ; 13C NMR (125.76 MHz, CDCl3) 16.1, 45.6, 106.1, 116.3, 124.2, 124.7, 125.0, 126.5, 128.8, 130.0, 133.3 and 143.9; HRMS: MH+ , found 143.0724. C12H10N requires 168.0813.
Procedure for the synthesis of 2-Amino-4-methylpyrimidine 18: A mixture of sodium ethoxide (2.06g, 30.3mmol) and guanidine nitrate (1.38g, 11.3mmol) in ethanol was stirred for 20minutes. Then a solution of butanone 1 (1g, 7.57mmol) was added and the reaction mixture was refluxed for 6h (TLC). The solvent was removed under reduced pressure and the reaction mixture was treated with water and extracted with ethyl acetate, washed (H2O), dried (Na2SO4) and the solvent was removed under vacuum to get the crude pyrimidine 18 which was passed over silica gel column eluting with petroleum ether and ethyl acetate (94:6) to yield the colorless crystalline needles in 66% yield. [Found: mp 150-152 °C; IR (Liquid film) 3297, 3154, 3001, 2154, 1563,1211cm-1 ; 1H NMR (400 MHz CDC13) 2.33 (3H, s, CH3), 5.01 (2H, s, NH2), 6.48 (1H, d, J=5.2 Hz, ArH), 8.13 (1H, d, J=4.8Hz, ArH) ; 13C NMR (99.5 MHz, CDCl3) 24.1, 111.4, 158.0, 163.0, 168.5; HRMS: MH+ , found 110.0717. C5H8N3 requires 110.0718.
Procedure for the synthesis of 2-Benzylamino-4- methylpyrimidine 19: To a well stirred suspension of sodium ethoxide (2.06g, 30.3mmol) in ethanol and benzyl guanidine sulfate (2.8g, 11.36mmol) at room temperature for 20minutes. A solution of butanone 1 (1g, 7.57mmol) in ethanol was added and the reaction mixture was refluxed for 6h (TLC). The reaction mixture after work up with cold water and extraction with ethyl acetate, washed (H2O), dried (Na2SO4) and evaporated to yield the corresponding crude pyrimidine 19 which was passed through silica gel column and eluting with petroleum ether and ethyl acetate (94:6) to yield the colorless crystalline needles in 58% yield. [Found: mp 70-72 °C; IR (solid film) 3271, 3030, 2920, 1948, 1567 cm-1 ; 1H NMR (400 MHz CDC13) 2.31 (3H, s, CH3), 4.62 (2H, d, J=5.96 Hz, CH2), 5.48 (1H, s, NH), 6.41 (1H, d, J=5.04 Hz, ArH), 7.35-7.22 (5H, m, ArH), 8.10 (1H, d, J=4.56 Hz, ArH); 13C NMR ((99.5 MHz, CDCl3) 24.2, 45.4, 45.5, 110.6, 127.2, 127.6, 128.6, 139.4, 157.7, 162.3, 168.1; HRMS: MH+, found 200.1114. C12H14N3 requires 200.1188.
Conclusion
The 4, 4-Dimethoxy-2-butanone is commercially available and consequently the chemistry based on its reactivity has been reported sparingly and the present communication explains some useful important reactions for the synthesis of aromatic and heteroaromatic compounds.
Acknowledgements
H.J. is grateful to Indian National Science Academy (INSA) for the award of Honorary Scientist. We gratefully acknowledge the CSIR, New Delhi (02(0106)/12 EMR-II Dated 01/11/12) for the financial support through the project. We are grateful to Prof. J N Moorthy, Professor, Indian Institute of Technology, Kanpur for generous help in providing Analytical Service. The authors thank Dr. P Shyamaraju, Chancellor, REVA University, Yelahanka, Bengaluru-560 064, for laboratory facilities.
Dedicated to Prof Henry J Shine, Paul Whitfield Horn Professor, Emeritus, Texas Tech University, Lubbock, USA on his 94th Birthday.
References
- Burness DM (1956) β-Keto acetals. I. synthesis of pyrazoles and pyrimidines and the steric inhibition of resonance in 5-alkyl-1-pnitrophenylpyrazoles. J Org Chem 21(1): 97-101.
- Bin L, Lin C, Xiaoxi F, Junwu Y, Lanfeng B, et al. (2015) Preparation of thiazole derivatives as acaricides. Patent, Faming Zhuanli Shenqing, CN 104649997, p. 33.
- Bin L, Lin C, Xiaoxi F, Junwu Y, Lanfeng B, et al. (2015) 2,4- Dimethyloxazolyl-acrylonitrile compounds as pesticides and their preparation, pharmaceutical compositions and use in control of pests and mites. Patent, Faming Zhuanli Shenqing, CN 104650063, p. 34.
- Huibin Y, Bin L, Cong F, Yanfeng C, Bin W, et al. (2014) Preparation of acrylonitrile compounds as agrochemical pesticides and acaricides. Patent, Faming Zhuanli Shenqing, CN 103833668, pp. 41.
- Huibin Y, Bin L, Yuquan S, Yanfeng Z, Bin W, et al. (2014) Preparation of acrylonitrile compounds as agrochemical insecticides. Patent, PCT International Application, WO 2014079354, pp. 61.
- Nielsen CB, Arnbjerg J, Johnsen M, Jorgensen M, Ogilby PR (2009) Molecular tuning of phenylene-vinylene derivatives for two-photon photosensitized singlet oxygen production. Journal of Organic Chemistry 74(23): 9094-9104.
- Butterfield AM, Gilomen B, Siegel JS (2012) Kilogram-scale production of corannulene. Organic Process Research and Development 16(4): 664- 676.
- Kelber C (1910) Über die elinwirkung von schwefelkohlenstoff und atzkali auf acetophenon. European Journal of Inorganic Chemistry 43(2): 1252-1259.
- Kelber C, Schwarz A (1911) Über die einwirkung von schwefelkohlenstoff und ätzkali auf p‐tolyl‐ und auf α‐thienyl‐methylketon. Berichte der Deutschen Chemischen Gesellschaft 44(2): 1693-1700.
- Kelber C, Schwarz A (1912) Die konstitution der desaurine. European Journal of Inorganic Chemistry 45 (1): 137-147.
- Bekington M, Hiriyakkanavar I, Junjappa H (1983) Polarized ketene dithioacetals, a new general highly stereo selective and regiospecific method for homologation of ketones to α, β-unsaturated esters via α-oxoketene dithioacetals. American Chemical Society, Journal of Organic Chemistry 48(26): 5327-5332.
- Dieter RK (1986) α-Oxo ketene dithioacetals and related compounds, versatile three-carbon synthons. Tetrahedron 42(12): 3029-3096.
- Junjappa H, Ila H, Asokan CV (1990) α-Oxoketene-S, S-, N, S- and N, N acetals: Versatile intermediates in organic synthesis. Tetrahedron 46(16): 5423-5506.
- Kolb M (1990) Ketene dithioacetals in organic synthesis: Recent developments. Thieme, Synthesis 1990(3): 171-190.
- Junjappa H, Ila H (1994) α-Oxoketene dithioacetals as intermediates for aromatic annelation. Phosphorus, Sulfur and Silicon and the Related Elements. Indian Academy of Sciences 95(1-4): 35-54.
- Katritzky AR, Li J, Xie L (1999) [3+3] Benzannulations of benzenoid- and heteroaromatic-ring systems. Tetrahedron 55(28): 8263-8293.
- Ila H, Junjappa H, Barun O (2001) Studies on regioselective addition of benzylic organometallics to α-oxoketene dithioacetals in our aromatic annelation protocol. Journal of Organometallic Chemistry 624(1-2): 34- 40.
- Ila H, Junjappa H, Mohanta PK (2001) The Junjappa-Ila (JI)- heteroaromatic annulation: a new general α-oxoketene dithioacetals mediated inverse method for the synthesis of benzo/condensed heterocycles and related hetero aromatization processes. Progress in Heterocyclic Chemistry 13(1): 1-24.
- Singh G, Ila H, Junjappa H (1984) Cationic benzoannelation of active methylene ketones via oxoketendithioacetals. Elsevier, Tetrahedron Letters 25(44): 5095-5098.
- Cathy E, Luche JL (1987) Selective allylation of carbonyl compounds in aqueous media. Journal of Organometallic Chemistry 322(2): 177-183.
- Christian P, Louche JL (1985) Allylzinc reagents additions in aqueous media. J Org Chem 50(6): 910-912.
- Rao CS, Balu MP, Ila H, Junjappa H (1991) Cycloaromatization of α- oxoketene dithioacetals and β-oxodithioacetals with benzyl-1- (naphthylmethyl) and 2-(naphthylmethyl) magnesium halides: synthesis of condensed polynuclear aromatic hydrocarbons. Elsevier, Tetrahedron, 47(20-21): 3499-3510.
- Nandi S, Panda K, Suresh JR, Ila H, Junjappa H (2004) α-Oxoketene dithioacetal mediated aromatic annulation: highly efficient and concise synthetic routes to potentially carcinogenic polycyclic aromatic hydrocarbons. Tetrahedron. 60(16): 3663-3673.
- Chauhan SMS, Junjappa H (1974) The Use of α-ketoketene S, S-diacetals for a novel pyrimidine synthesis. Synthesis 12: 880-882.
- Chauhan SMS, Junjappa H (1976) Ketene-S, S-acetals-V: The reactions of α-keto and α-cyanoketene-S, S-acetals with guanidine and thiourea:a new general synthesis of alkoxy-pyrimidines. Tetrahedron 32(14): 1779-1787.
- Chauhan SMS, Junjappa H (1976) Ketene-S, S, -acetals-VI: Synthesis of 3,3-bis-(methylthio)-2-propene-2-alkyl-1-aryl-1-ones and their reaction with guanidine: A novel route for pyrimidine synthesis. Tetrahedron 32(15): 1911-1916.
- Kumar A, Aggarwal V, Ila H, Junjappa H (1980) A Novel and convenient synthesis of 2-amino-4-(N-alkyl-N-arylamino)-pyrimidines using polarized ketene S, S- and S, N-acetals. Synthesis 9: 748-751.
- Singh LW, Gupta AK, Ila H, Junjappa H (1984) A facile synthesis of 2-amino-4-alkoxy-6-styryl- and 2-amino-4-alkoxy-6-(4-aryl-1,3- butadienyl)-pyrimidines by direct cyclocondensation. Synthesis 6: 516- 518.
© 2018 Junjappa H. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






