- Submissions

Full Text
Orthopedic Research Online Journal
Comparative Ultrasonograhic Evaluation of Chemical Castration Using Graded Formalin and Physical Castration in Red Sokoto Bucks
Bodinga HA1*, Abdulsalam J, Umar A2, Abubakar N1, Buhari S1, Abdul AA1, Yakubu AS1, Bello A3
1Department of Veterinary Surgery and Radiology, Usmanu Danfodiyo University Sokoto, Nigeria
2Department of Theriogenology and Animal Production, Usmanu Danfodiyo University Sokoto, Nigeria
3Department of Veterinary Anatomy, Usmanu Danfodiyo University Sokoto, Nigeria
*Corresponding author: Bodinga HA, Department of Veterinary Surgery and Radiology, Usmanu Danfodiyo University Sokoto, Nigeria
Submission: May 28, 2020;Published: June 19, 2020

ISSN: 2576-8875 Volume7 Issue2
Abstract
This study aimed to determine the effectiveness of chemical castration using graded formalin concentration compared to surgical and bloodless castration with burdizzor in Red Sokoto bucks using ultrasonography. Eight healthy Red Sokoto bucks divided into four groups; A, B, C and D with average weight and age of 14kg and 10 months were used in this study. Group A, B, and C were castrated using 2.5mls of formalin each at different concentrations 2%, 4% and 6% injected bilaterally into the tail of the Epididymis while group D were castrated using surgical and bloodless castration using burdizzor respectively. The sonogram of the testicles was taken before and after chemical and bloodless castration using burdizzor to determine the effectiveness and level of testicular degeneration. The bucks were sedated using xylazine at 0.05mg/kg and atropine an ant cholinergic at 0.2mg/kg body weight intramuscularly. The sonogram of the testicular parenchyma of the bucks injected with formalin appears hyper echoic indicating testicular degeneration while the sonogram of testicular parenchyma of the buck castrated using burdizzor appear Anaechoic (indicating fluid accumulation) and areas of high echogenicity. From this study, formalin can be an effective chemical castration agent in Small Ruminants.
Keywords: Castration; Ultrasonography; Formalin; Red sokoto goat; Comparative study
Introduction
The Goat (Capra aegagrus hircus) is a subspecies of goat domesticated from the wild goat (Capra aegagrus) [1]. Red Sokoto goat is an indigenous breed of goat common to the Northern region of Nigeria [2]. Goats provide meat, milk, hides and skin and manure. In the small ruminant sector, goats are one of the most essential food-producing animal species in developing countries and an integral part of a traditional crop-livestock production [3]. In Nigeria, goats are kept by rural areas in small herds to serve as sources of financial stability, a supply of meat and security against crop failure. The castration of male goats is a routine practice in many countries aimed at stopping the production of male hormones and sperms, preventing mating after the age of puberty, producing animals to be easier to handle with less aggressiveness and improving meat quality [4]. Also to avoid unwanted pregnancies and mating of young females before they are of adequate size and age for pregnancy and parturition and reduce goat smell in males [5]. There are various methods of castration used in animals, which include physical, hormonal and chemical methods [6].
The physical methods involve surgical removal of testicles, application of rubber ring at the base of the scrotum and bloodless castration by using burdizzor clamps. Hormonal techniques it involves the injection of immuno-contraceptive to induce antibody production against gonadotropin-releasing factor leading to decreased production of an endogenous hormone [7]. Chemical castration involves injecting a sclerosing or toxic agents into testicular parenchyma to cause irreversible damage and functional loss [7].
Many researches has been carried out with the use of Ultrasonography as modern diagnostic tools in medicine to visualize many internal organs, their size, structure and any pathological lesions with real-time tomographic images. Transcutaneous ultrasonography is used to demonstrate the ultrasonic morphology of testes of bulls rams and boars to detect the level of tissue degeneration through intra-testicular injection of chemical agents such as ferric chloride, BCG [8] glycerol [9], Chlorhexidine and cottonseed oil [10] to achieve the desired goal of castration with little information on comparative ultrasonographic changes using graded formalin and physical castration in red Sokoto bucks.
This study will provide information on the ultrasonographic changes of testes of Red Sokoto bucks following intra testicular injection of graded concentration of formalin. It will be useful for the detection of the level of tissue degeneration. It will also be useful in preventing intra operative and postoperative complications of surgical method and the cost of medication. The results obtained from this research will be useful for the clinicians as it is easy and reliable and will also be useful for teaching in diagnostic imaging and contribute to the existing literature. This study aimed to determine the effectiveness of chemical castration using graded formalin compared to surgical castration and bloodless castration using burdizzor in Red Sokoto buck using ultrasonography.
Materials and Methods
The was carried out in the Department of Surgery and Radiology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto. Sokoto State is geographically located in the North-Western part of Nigeria between longitude 11º 30º to 13º 50º East and latitude 4º to 6º 40º North. The state shares common borders with the Niger Republic to the North, Kebbi State to the South, and Zamfara State to the East. The state falls in the dry Sahel surrounded by sandy Sudan type Savannah. The state, a major livestock producer lying in the arid region of the country, covers a total land area of about 32,000 square km with an estimated human population of 3,696,999 million [11].
Eight healthy Sokoto Red bucks weighing 12-15kg and aged between 6 months to 1 year were purchased from the reputable livestock market in Bodinga, Bodinga Local Government area of Sokoto State, Nigeria and used in the study. Upon purchase they were de-wormed with albendazole at a dose rate of 7.5mg/kg body weight orally, 20% ox tetracycline at 10mg/kg body weight and fed with groundnut hay, bean husks and water provided adlibitum for two weeks before the study.
The bucks were divided into four different groups; A, B, C and D with two animals in each group. Group A, B, and C were castrated using a chemical method, 2%, 4% and 6% formalin (2.5mls) were injected intra-testicular bilaterally in groups A, B, and C respectively. Before injection, the animals were sedated using xylazine at 0.5mg/kg body weight and atropine at 0.02mg/kg body weight intramuscular. Pre-injection ultrasonographic evaluation of all the testes was carried out and subsequently, ultrasonographic Evaluation was performed at day 7, 14 and 21 respectively all changes in testicular tissue were observed and recorded. Group D animals, the control group were castrated using surgical and bloodless castration using burdizzor respectively in D1 and D2.
Surgical procedure
The bucks were positioned on dorsal recumbency and the scrotal and prescrotal are aseptically prepared for surgery. Two parallel incisions (1-1.5cm each) were made through the skin at the prescrotal area over the spermatic cord, using blunt dissection each spermatic cord was exteriorized over an artery forceps. After careful incision of tunica virginalis, the vas deference was identified then bluntly separated, a segment of about 2cm of the vascular portion of spermatic cord was clamped by using two artery forceps then apply the cautery device in between until complete separation, the two ends of blood vessels were checked for bleeding before reposition Skin incision was closed by Ford interlocking suture pattern.
Bloodless castration procedure using burdizzor
The bucks were restrained with the hind limbs held apart and scrotal area exposed for correct application of the Burdizzor castrator. The instrument was applied laterally onto the scrotal neck behind the goat. The cord was held laterally in the scrotal neck by first finger and thumb, with the second hand directing the position of the jaws slowly, until they were about 8-10mm apart to grip the skin and cord firmly. Rapid closure was carried out and maintained for 15-30 seconds, during which the cord was correctly crushed as described by Olaifa [12]. Ultrasonographic evaluation of the testicular tissue following chemical and bloodless castration using burdizzor continues subsequently at days 7, 14 and 21. The level of testicular damaged was observed and recorded in all the groups.
Results and Discussion
Figure 1A & B: (Longitudinal) Showing normal sonographic appearance of (1) Testicular parenchyma (homogenous), (2) Mediastinum and (3) Tunica vaginalis.

Day 0: Pre-ultrasonographic Evaluation showing normal testicles. (Figure 1)
Day 7: After injection of the chemical agent and close castration using Burdizzo showing Sonographic appearance of Testicular parenchyma appearing anaechoic and areas of increase echogenicity. (Figure 2)
Day 14: After injection of the chemical agent and close castration using burdizzor showing Sonographic appearance of Testicular parenchyma appearing anaechoic and areas of increase echogenicity. (Figure 3)
Day 21: After injection of the chemical agent and after close castration using burdizzor. (Figure 4)
Day 28: After injection of the chemical agent and close castration using burdizzor. (Figure 5)
Based on the result of this study it was observed that surgical methods of castration have remained the major existing method of castration used in bucks but accompanied by many disadvantages and post-operative complications such as hemorrhage and dehiscence. This observation is similar to a report by Adin [13].
Figure 2A-D: Sonogram showing increased echogenicity (hyperechoic) of the Testicular parenchyma Key:- Group A: 2% formalin injected bilaterally into the tail of the epididymis, Group B: 4% formalin injected bilaterally into the tail of the epididymis, Group C: 6% formalin injected bilaterally into the tail of the epididymis and Group D: Bloodless castration using burdizzor.

Figure 3A-D : showing sonographic appearance of Testicular parenchyma appearing anaechoic and areas of increase echogenicity Key:- Group A: 2% formalin injected bilaterally into the tail of the epididymis, Group B: 4% formalin injected bilaterally into the tail of the epididymis, Group C: 6% formalin injected bilaterally into the tail of the epididymis and Group D: Bloodless castration using Burdizzor.

Figure 4A-D: Sonographic appearance of Testicular parenchyma with increased echogenicity (Hyperechoic) Key:- Group A: 2% formalin injected bilaterally into the tail of the epididymis, Group B: 4% formalin injected bilaterally into the tail of the epididymis, Group C: 6% formalin injected bilaterally into the tail of the epididymis and Group D: Bloodless castration using Burdizzor.
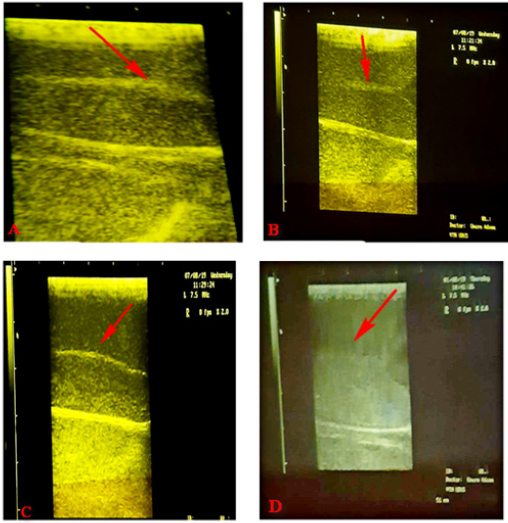
Figure 5A-D: Sonographic appearance of Testicular parenchyma with increased echogenicity (Hyperechoic) Key:- Group A: 2% formalin injected bilaterally into the tail of the epididymis, Group B: 4% formalin injected bilaterally into the tail of the epididymis, Group C: 6% formalin injected bilaterally into the tail of the epididymis and Group D: Bloodless castration using Burdizzor.

In close castration using burdizzor, the sonogram appears anechoic indicating fluid accumulation with slight hyper echoic areas indicating testicular parenchyma degeneration. This finding is similar to that of Ahmad et al. [14] who described a case of hydrocoele in a male goat following ligation of the testicular artery. The affected testis was encircled by an anechoic fluid with hyper echoic fibrin strands within the fluid. Also similar to report by Ahmad [15] & Ahmad et al. [16] who observed anechoic areas, representing sperm granulomata within the epididymis head and the tail of infertile rams. This is also in line with the findings by Ali et al. [17] infertile bulls who presented bigger testes, pulpy consistency with many anechoic areas within the testis and the epididymis head, demarcated from the rest of the ultrasonographic measurement.
In chemical Castration using graded formalin, the testicular parenchyma appears hyper echoic indicating testicular degeneration. This finding is in line with the report of Ali et al. [17] who reported that an infertile bull presented hard consistency of the testes and abundance of hyper echoic areas with acoustic shadowing scattered in the parenchyma of both testes which were assumed to be mineralization in the testes. Similarly; Ahmad et al. [18] & Ahmad [15] observed similar hyper echoic areas in the testicular parenchyma of infertile goats. These findings also agree with the findings of Ali et al. [17] in Brucella affected infertile bulls, the tail of the epididymis enlarged with few hyper echoic areas and acoustic shadowing, presumably representing chronic Epididymis. Also, this finding corresponds to findings by Ahmad et al. [19] who described similar observations in a ram in which the tail of epididymis revealed a few hyperechoic areas.
Conclusion
Based on the findings of this study, it can be concluded that formalin can be used as a chemical agent for castration in small ruminants. Chemical castration is effective and causes less stress compared to physical castration. The intra and post-operative complications are disadvantages associated with surgical castration
Recommendation
It is recommended that 4% formalin (chemical castration) should be used as an alternative to veterinary practitioners to overcome the challenges of uncertainty in close castration using burdizzor and also the complications associated with surgical castration.
References
- Yakubu A, Salako AE, Imumorin IG, Ige AO, Akinyemi MO (2010) Discriminant analysis of morph metric differentiation in the West African Dwarf and Red Sokoto goats. South African Journal of Animal Science 40(4): 381-387.
- Ngere LO, Adu IF, Okubanjo IO (1984) The indigenous goats of Nigeria. Animal Genetic Resources Information 3: 1-9.
- Seyoum S (2002) Economic of small ruminant meat production and consumption in Sub Saharan Africa. International Livestock Centre for Africa 2: 15-26.
- Molony V, Kent JE, Robertson IS (1995) Assessment of acute and chronic pain after different methods of castration of calves. Appl Anim Behav Sci 46: 33-48.
- Burciage RL, Step DL, Holland BP, McCurdy MP, Krehbiel CR (2006) Castration in goats: Technique and animal welfare issues. Compend Cont Educ Pract Veterinarian 24(9): 512-515.
- Rajkumar D (2013) Minimization of pain in cattle castration with respect to method, age and pain relief.
- Fordyce G, Hodge PB, Beaman NJ, Laing AR, Campero C, et al. (1989) Evaluation of calf castration by intra testicular injection of lactic acid solution. Australian Veterinary Journal 66: 272-276.
- Das RP, Mustafa AS, Talwar GP (1982) Atrophy of seminiferous tubules of mouse testes after intra testicular injection of BCG and their regeneration. Archive of Andrology 9(3): 244-250.
- Immegart HM, andThrelfall WR (2000) Evaluation of intra-testicular injection of glycerol for non- surgical sterilization of dogs. Journal of American Veterinary Research 61(5): 544-549.
- Fadason ST (2001) Effects of Intra testicular Injection of Chlorhexidine Gluconate and Cottonseed Oil on Some Reproductive Parameters of Bucks. Department of Veterinary Surgery and Medicine, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria.
- Alkali BR, Shuaibu AB, Bello I, Usman MD (2017) Seroprevalence of newcastle disease virus in local chickens from Sokoto, Nigeria. Microbiology Research Journal International 19(1): 1-5.
- Olaifa AK, Opara MN (2011) Hematological and biochemical parameters of West African dwarf bucks castrated by the Burdizzor method. Vet Archive 81: 743-750.
- Adin CA (2011) Complications of ovariohysterectomy and orchiectomy in companion animal. Vet Clin Small Animalm 41: 1023-1039.
- Ahmad N, Samad HA, Rehman NU, Ahmad KM, Ahmad M (1999) An ultrasonographic and histopathological study of the testis and epididymides following experimentally induced unilateral ischemia in male goats and rams. Pakistan Veterinary Journal 19: 204-209.
- Ahmad N, Noakes DE (1995) A clinical and ultrasonographic study of the testes and related structures of goats and rams after unilateral vasectomy. Veterinary Record 137: 112-117.
- Ahmad N, England GCW, Noakes DE (2000) Ultrasonography of spontaneous lesions of the genital system of three rams, and their influence on semen quality. Veterinary Record 146: 10-15.
- Ali KM, Ahmad N, Akhtar N, Ali S, Ahma M, Younis M (2011) Ultrasound imaging of testes and epididymides of normal and infertile breeding bulls. Pakistan Veterinary Journal 31(4): 345-350.
- Ahmad N, Noakes DE, Middleton DJ (1993) Use of ultrasound to diagnose testicular degeneration in a goat. Veterinary Record 132: 436-439.
- Ahmad N, Noakes DE, Subandro AL (1991) B-model real-time ultrasonographic imaging of testes and epididymides of sheep and goats. Veterinary Record 128: 491-496.
- Fisher AD, Crowe MA, Alonso de la Varga Me, Enright WJ (1996) Effect of castration method and the provision of local anesthesia on plasma cortisol, scrotal circumference, growth and feed intake in bull calves. Journal of Animal Science 74: 2336-2343.
© 2020 Bodinga HA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






