- Submissions

Full Text
Medical & Surgical Ophthalmology Research
Comparison of Intravitreal Ranibizumab and Bevacizumab Treatment for CNV
Won Seok Choi, Dong Wook Kim, Chi Shian Feng, Sang Joon Kim, Ha Kyoung Kim and Jae Ryong Han*
Department of Ophthalmology, Hallym University College of Medicine, Korea
*Corresponding author: Jae Ryong Han, Department of Ophthalmology, Hallym University College of Medicine, Dongtan Sacred Heart Hospital 40, Seoku-dong, Hwaseong-si, Gyeonggi-do, Korea
Submission: June 14, 2018;Published: June 20, 2018

ISSN 2578-0360 Volume2 Issue3
Abstract
Purpose: To compare the efficacy of bevacizumab and ranibizumab in the treatment of patients with choroidal neovascularization (CNV).
Methods: Patients who received anti-vascular endothelial growth factor (VEGF) agents for CNV. Best-corrected visual acuity (BCVA) and central macular thickness (CMT) were used to compare treatment outcome. BCVA and CMT were by dividing into 3 subgroups; idiopathic CNV, CNV with agerelated macular degeneration (AMD), and polypoidal choroidal vasculopathy (PCV).
Result: In total CNV patients, both anti-VEGFs showed improvement in BCVA and CMT but they did not show difference in changes of BCVA and CMT. In idiopathic CNV, both anti-VEGFs showed improvement in BCVA and CMT but they did not show difference in changes of BCVA and CMT. In AMD, BCVA and CMT were improved only at 3 months after injection and did not show difference in changes of BCVA and CMT. In PCV, BCVA of patients who received bevacizumab improved through the whole study period, otherwise that of patients who received ranibizumab improved only at 3 months later. CMT of patients with PCV had improved until 3 months after injection in both bevacizumab and ranibizumab group.
Conclusion: Intravitreal bevacizumb injection showed similar efficacy and safety as ranibizumab in CNV patients with various pathologic conditions.
Keywords: Choroidal neovascularisation; Bevacizumab; Ranibizumab
Abbreviations: CNV: Choroidal Neovascularization; VEGF: Vascular Endothelial Growth Factor; BCVA: Best-Corrected Visual Acuity; CMT: Central Macular Thickness; AMD: Age-Related Macular Degeneration; PCV: Polypoidal Choroidal Vasculopathy; PDT: Photodynamic Therapy; OCT: Optical Coherence Tomography
Introduction
Choroidal neovascularization (CNV) is defined as growth of new blood vessels from the choriocapillaris into the subretinal space or subretinal pigment epithelium [1,2]. CNV causes visual loss by macular exudation, hemorrhage and fibrosis [3]. CNV is a common pathologic condition that occurs in many chorioretinal diseases such as age-related macular degeneration (AMD), pathologic myopia, angioid streak, sarcoidosis, histoplasmosis, chroidal nevus, melanoma, choroidal rupture, polypoidal choroidal vasculopathy (PCV) and idiopathic causes [2,4]. Among these diseases AMD and PCV is very common, in Asia, it represents between 56 and 72 % of all neovascular AMD patients [4]. Various therapeutic options, including photodynamic therapy (PDT) [5-7], anti-vascular endothelial growth factor (VEGF) agents [8-10], have been reported to manage CNV. Recently, pegaptanib, bevacizumab and ranibizumab are used as an anti-VEGF agent [11].
Among these agents, bevacizumab and ranibizumab are commonly used in Korea. Ranibizumab, a high-affinity recombinant humanized antigen-binding Fob fragment which neutralizes active isoforms of VEGF-A [12]. However, bevacizumab, full-length recombinant humanized antibody binding to all VEFG isoforms, was developed to for treatment of metastatic colorectal cancer and is not approved by Food and Drug Administration for ophthalmic disease [12]. Nevertheless, bevacizumab is used as off-label drug by worldwide ophthalmologist for the treatment of CNV [13,14] because it has been reported that bevacizumab has favorable efficacy and safety profiles and costs lower than ranibizumab [10,14-28]. In this study, the effects of intravitreal bevacizumab injection and ranibizumab injection commonly used in managing CNV were compared and analyzed by dividing into 3 subgroups ; idiopathic CNV, CNV with AMD, and PCV.
Methods
A retrospective review of patients with CNV was performed. Patients who were treated with bevasizumab or ranibizumab for more than 6 months from January 1, 2008, to June 1, 2011, were included. From August 1, 2009 in Korea, ranibizumab began to be covered by national health insurance so many patients who had CNV were begun to treated with ranibizumab. Patients were excluded who had any other treatment such as photodynamic therapy (PDT), intravitreal triamcinolone injection or surgery within 6 months. All patients diagnosed by undergoing optical coherence tomography (OCT), fluorescein angiography, indocyanine green angiography. At each follow-up visits best-corrected visual acuity (BCVA) and OCT were performed. BCVA was measured by snellen chart and converted to logarithm of the minimum angle of resolution (log MAR).
OCT was used to quantify central macular thickness (CMT). From January 1, 2008 to August 31, 2009, Stratus OCT (Carl Zeiss Meditec, USA) was used and after then Cirrus OCT (Carl Zeiss Meditec, USA) was used. To minimizes the bias from changing of model of OCT during the study period, 50㎛ was added to CMTs measured by Stratus OCT [29-31]. Both intravitreal bevacizumab and ranibizumab injections were performed with Pro re Nata. Patients received an injection only if the OCT shows a recurrence of fluid or hemorrhage. Intravitreal injections were performed under sterile condition at operating room and before injection the eye was administered topical anesthesics (Alcaine®; Alcon, USA). After then 5% povidone-iodine solution was used to disinfect lids and lashed, and a sterile speculum was placed. The ocular surface was irrigated with 5% povidone-iodine solution and washed out with 5% normal saline. Bevacizumab (1.25mg/0.05ml) or ranibizumab (0.5mg/ 0.05ml) was injected with 1-mL tuberculin syringe and 30 gauge needle through the pars plana 3.5mm inferotemporally posterior to the limbus. After injection topical anti-biotics (Vigamox®; Alcon, USA) was prescribed to use 4 timed a day for a week.
Analyses were performed using SPSS software, version 12.0K (SPSS Inc., Chicago, IL, USA). This study was approved by the Institutional Review Board of Hallym University Medical Center. All subjects signed an informed consent form before participating in the study.
Results
48 eyes were included in the bevacizumab group and 41 eyes in the ranibizumab group. The mean age of bevacizumab group was 56±18 and that of ranibizumab group was 68±8. Both groups were divided into three subgroup; idiopathic CNV, AMD and PCV. In bevacizumab group, number of idiopathic CNV patients was 24, that of AMD patients were 13 and that of PCV patients were 11. In ranibizumab group, number of idiopathic CNV patients was 15, that of AMD patients were 14 and that of PCV patients were 12 (Table 1). There was no significant difference between the numbers of injection within both groups. (p = 0.05, independent sample t-test) The number of injections in bevacizumab group was 3.08±0.74 and that in ranibizumab group was 3.39±0.74, also showed no significant difference (p = 0.372, Mann-Whitney U test).
Table 1:Demographics of patients.
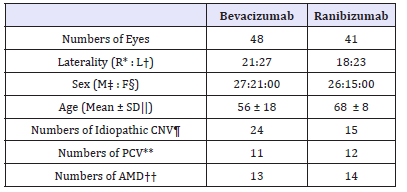
R*: Right, L†; Left, SD||: Standard Deviation; CNV¶: Choroidal Neovascularization; PCV**: Polypoidal Choroid Vasculopathy; AMD†† : Age-related Macular Degeneration
Total CNV patients group
In bevacizumab group, baseline BCVA was 0.61±0.45 and 0.70±0.46 in ranibizumab group. In bevacizumab group, BCVA was improved 1 month, 2 months, 3 months and 6 months later (p 0.001, p 0.001, p 0.001, p 0.001, respectively, paired sample t-test). Ranibizumab group also showed improvement of BCVA continuously (p = 0.015, p = 0.001, p = 0.001, p = 0.001, respectively, paired sample t-test).
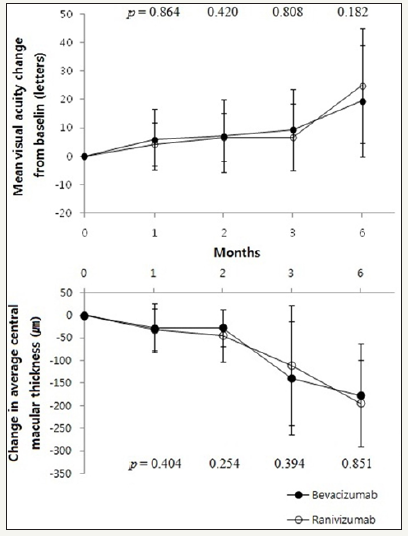
CMT in bevacizumab group significantly decreased than baseline at all 4 time points evaluated (p 0.001, p 0.001, p 0.001, p = 0.011, respectively, paired sample t-test) and same trend appeared in ranibizumab group (p 0.001, p 0.001, p 0.001, p = 0.028, respectively, paired sample t-test) (Table 2). Changes of BCVA and CMT did not show significant difference (Figure 1).
Table 2:Demographics of patients.
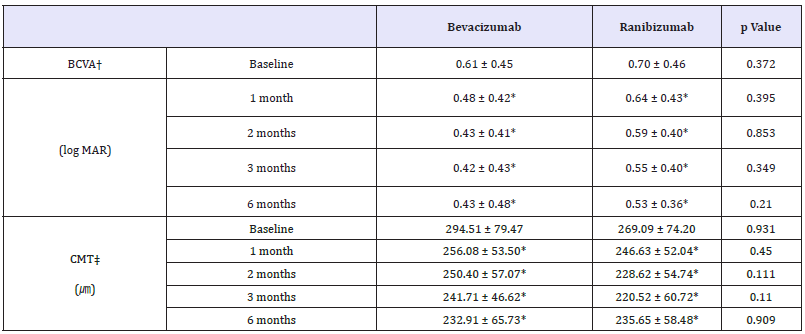
*p-value by paired sample t-test < 0.05 compared with baseline, BCVA†: Best-corrected visual acuity; CMT‡ : Central macular thickness
Figure 1:Mean visual acuity and central macular thickness change from baseline in total CNV patients.
Idiopathic CNV subgroup
The baseline BCVA was 0.58±0.47 in bevacizumab group and 0.77±0.57 in ranibizumab group. In bevacizumab group, BCVA was improved 1 month, 2 months, 3 months and 6 months later (p = 0.002, p = 0.001, p = 0.001, p = 0.001, respectively, Wilcoxon signed rank test). Ranibizumab group also showed improvement of BCVA continuously (p = 0.015, p = 0.010, p = 0.016, p = 0.009, respectively, Wilcoxon signed rank test).
CMT in bevacizumab group significantly decreased than baseline at all 4 time points evaluated (p = 0.001, p = 0.002, p = 0.002, p = 0.028, respectively, Wilcoxon signed rank test) and same trend appeared in ranibizumab group (p = 0.005, p = 0.004, p = 0.004, p = 0.043, respectively, Wilcoxon signed rank test) (Table 3). Compared with the baseline, the changes in BCVA, as well as the changes in CMT, between bevacizumab group and ranibizumab group did not show any significant difference (Figure 2).
Table 3:Changes in visual acuity and central macular thickness after injection in idiopathic CNV subgroup.
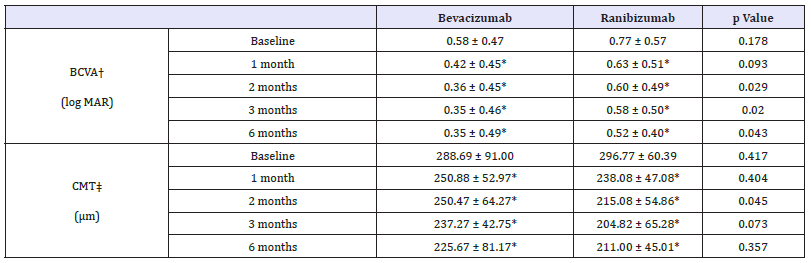
*p-value by paired sample t-test < 0.05 compared with baseline, BCVA†: Best-Corrected Visual Acuity; CMT‡: Central Macular Thickness
Figure 2:Changes in visual acuity and central macular thickness after injection in idiopathic CNV patients.
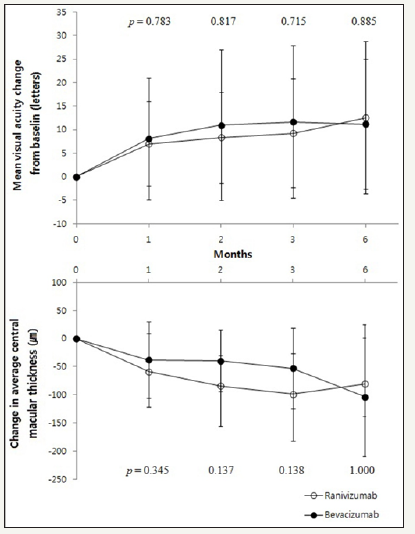
AMD subgroup
In bevacizumab group, baseline BCVA was 0.59±0.44 and 0.56±0.43 in ranibizumab group. The BCVA in bevacizumab group was significantly improved at 3 months later (p = 0.030, Wilcoxon signed rank test) and did not at 1 month, 2 months and 6 months later (p = 0.161, p = 0.169, p = 0.116, respectively, Wilcoxon signed rank test). In ranibizumab group, BCVA was also significantly improved only at 3 months later (p = 0.022, Wilcoxon signed rank test) and did not at 1 month, 2 months and 6 months later (p = 0.893, p = 0.449, p = 0.307, respectively, Wilcoxon signed rank test) (Table 4). Compared with the baseline, the changes in BCVA, between bevacizumab group and ranibizumab group did not show any significant difference statistically (Figure 3).
Figure 3:Changes in visual acuity and central macular thickness after injection in AMD patients.
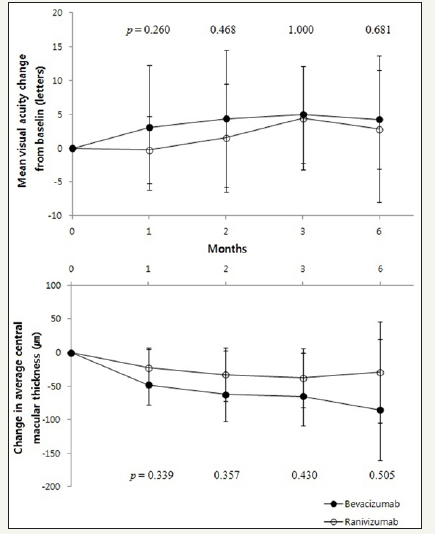
Table 4:Changes in visual acuity and central macular thickness after injection in AMD subgroup.
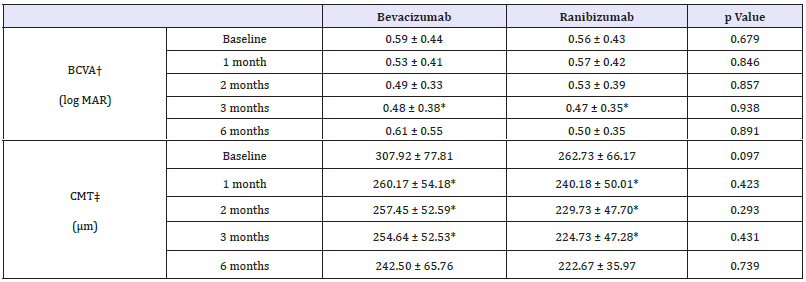
p-value by paired sample t-test < 0.05 compared with baseline, BCVA† : Best-corrected visual acuity, CMT‡ : Central macular thickness
CMT in bevacizumab group significantly decreased than baseline at 1 month, 2 months and 3 months later but not at 6 months later (p = 0.005, p = 0.013, p = 0.008, p = 0.208, respectively, Wilcoxon signed rank test). Same trend appeared in ranibizumab group (p = 0.008, p = 0.005, p = 0.003, p = 0.345, respectively, Wilcoxon signed rank test) (Table 4). Compared with the baseline, the changes in CMT, between bevacizumab group and ranibizumab group did not show any significant difference (Figure 3).
PCV subgroup
Figure 4:Changes in visual acuity and central macular thickness after injection in PCV patients.
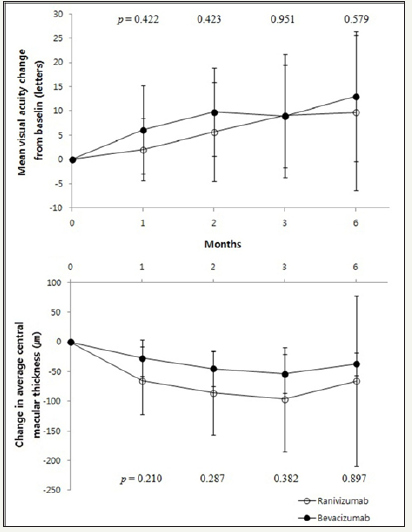
Table 5:Changes in visual acuity and central macular thickness after injection in PCV subgroup.
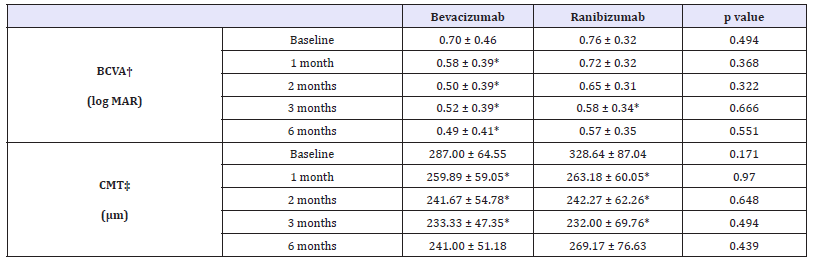
*p-value by paired sample t-test < 0.05 compared with baseline, BCVA†: Best-Corrected Visual Acuity; CMT‡: Central Macular Thickness
In bevacizumab group, baseline BCVA was 0.70±0.46 and 0.76±0.32 in ranibizumab group. The BCVA in bevacizumab group was significantly improved 1 month, 2 months, 3 months and 6 months later (p = 0.046, p = 0.011, p = 0.020, p = 0.035, respectively, Wilcoxon signed rank test). In ranibizumab group, BCVA was significantly improved only at 3 months later (p = 0.037, Wilcoxon signed rank test) and did not at 1 month, 2 months and 6 months later (p = 0.307, p = 0.100, p = 0.050, respectively, Wilcoxon signed rank test) (Table 5). Compared to the baseline, the changes in BCVA, between bevacizumab group and ranibizumab group did not show any significant difference statistically (Figure 4).
The CMT of bevacizumab group was decreased significantly at 1 month, 2 months and 3 months later after injection but not at 6 months later (p = 0.008, p = 0.008, p = 0.008, p = 0.109, respectively, Wilcoxon signed rank test). Ranibizumab group showed same trend that decreased until 3 months later but not at 6 months after injection (p = 0.010, p = 0.004, p = 0.003, p = 0.249, respectively, Wilcoxon signed rank test) (Table 5). Compared with the baseline, the changes in BCVA, between bevacizumab group and ranibizumab group did not show any significant difference statistically (Figure 4).
Discussion
Intravitreal anti-VEGF injection is one of the most common treatment for CNV patients in the world. It is more effective to prevent decreasing visual acuity by CNV than PDT [29]. Among many anti-VEGF drugs, ranibizumab and bevacizumab are the most commonly used. However, there are not enough prospective and case-control studies about the comparison between them.
As studies about ranibizumab, ANCHOR [30] and MARINA [31] trials has reported and long term follow-up studies are currently in progress. However bevacizumab is also important issue because it is more cost-effective than ranibizumab. Recently, CATT (US), IVAN (UK), VIBERA (German), LUCAS (Norway), GEFAL (France), EQUAL (Netherland) and MANTA (Austria) are is in progress currently to compare the effect between bevacizubam and ranibizumab [32,33- 37].
In this study, intravitreal bevacizumab treatment and ranibizumab showed no significant difference in improvement of visual acuity and resolution of increased macular thickness after injection in whole CNV patients group until 6 months. This results for 1 month after injection correlate closely with the findings from previous study that Biswas et al. investigated about the efficacy of bevacizumab and ranibizumab [38]. The result that increases of ETDRS letters in idiopathic CNV are higher than that of CNV with AMD is also similar to previous study [39]. In idiopathic CNV subgroup, both drugs showed improvement of visual acuity and CMT.
In AMD subgroup, both drugs showed improvement of CMT to 3 months but that of visual acuity from 3 months after treatment and after 6 months, visual acuity was decreased to the baseline level. Lastly, in PCV subgroup, the effect of improving BCVA was shown earlier in bevacizumab but effect of reducing CMT was similar and CMT was thickened again after 6 months in both treatment groups. In case of PCV, treatment is not exactly established and it is known that PDT is effective [40-42], therefore it is thought that only anti- VEGFs treatment has limitation.
Although ranibizumab is made for treatment of ophthalmic disease as anti-VEGF, bevacizumab is still used instead of ranibizumab by many clinicians. Recently, a lot of studies have been reported about efficacy of bevacizumab versus ranibizumab [20,23,38,43] but most studies are comparing only in AMD. While the previous studies compared the effects between bevacizumab and ranibizumab in AMD patients, we compared in CNV patients which can occurred in not only in AMD but also in PCV and by idiopathic courses.
Subramanian et al reported that the effects of both anti-VEGFs in AMD showed no difference in visual and anatomic outcomes [44]. However the degrees of changes in BCVA and CMT in this study differ from other reports. It is referable to the differences in characteristics of enrolled patient group.
Several adverse events related with intravitreal injection of anti-VEGF, such as elevated intraocular pressure, endophthalmitis, stroke and myocardial infarction have been reported before [20,32,45]. By the recently reported multicenter, randomized study Comparison of AMD Treatments Trials Ranibizumab-Bevacizumab Trial (CATT), endophthalmitis, uveitis, ocular-vessel occlusion or embolism, retinal detachment and vitreous hemorrhage can develop in less than 1% of patient [32]. In our study, however, such adverse events were not occurred, only several cases of subconjunctival hemorrhage were occurred which improved within a week without any treatment.
Bevacizumab was used under various protocols in previous studied because it was thought to have different effect and efficacy. However this study has the advantage that was conducted in same institute and both anti-VEGFs were injected under the same condition and same protocol.
Our study has limitation that the CMT was measured by two different OCT models, Cirrus HD OCT and Stratus OCT. Because Cirrus HD OCT is reported that measures the same retinal layer about 50μm thicker than Stratus OCT does [46-48], we added 50μm to thickness measured by Stratus OCT and this can make bias of analysis. Furthermore, this study is retrospective and recruited a little number of patients. In the future, Prospective and randomized controlled trials will be needed. However this study is not randomized controlled study, beginning of covering by national health insurance made subjects be devided randomly into bevacizumab and ranibizumab groups.
In spite of small size of subjects and retrospective study, this study shows intravitreal bevacizumb injection has similar efficacy and safety as ranibizumab injection in CNV patients with various pathologies.
Acknowledgment Disclosure
All authors have no financial interest regarding the subject matter of this manuscript.
References
- Green WR, Wilson DJ (1986) Choroidal neovascularization. Ophthalmology 93(9): 1169-1176.
- Gass JDM (1997) Stereoscopic Atlas of Macular Diseases Diagnosis and Treatment, (4th edn), St Louis, Mosby, USA.
- Cheema RA, Mushtaq J, Cheema MA (2011) Intravitreal bevacizumab as a primary treatment for idiopathic choroidal neovascularization. Middle East Afr J Ophthalmol 18(3): 220-223.
- Yannuzzi LA The Retina Atlas. St Louis, Mosby, 2010.
- Verteporfin in Photodynamic Therapy Study Group (2001) Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization-- verteporfin in photodynamic therapy report 2. Am J Ophthalmol 131(5): 541-560.
- Azab M, Boyer DS, Bressler NM, Bressler SB, Cihelkova I, et al. (2005) Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration 2-year results of a randomized clinical trial. Arch Ophthalmol 123(4): 448-457.
- Bressler NM (2001) Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol 119(2): 198-207.
- Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR, et al. (2004) Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351(27): 2805-2816.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, et al. (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355(14): 1419-1431.
- Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. (2006) Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 113(3): 363-372.
- Kim HW, Kim JL, Lee MH, Hyung Gon Yoo, Young Chung, et al. (2011) Combined Treatment of Photodynamic Therapy and Bevacizumab for Choroidal Neovascularization Secondary to Age-Related Macular Degeneration. Korean J Ophthalmol 25(4): 231-237.
- Ferrara N, Damico L, Shams N, Lowman H, Kim R (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26(8) :859-870.
- Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. (2006) Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 113(3): 363-372.
- Spaide RF, Laud K, Fine HF, Klancnik JM Jr, Meyerle CB, et al. (2006) Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina 26(4): 383-390.
- Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, et al. (2006) Intravitreal bevacizumab for the management of choroidal neovascularization in agerelated macular degeneration. Am J Ophthalmol 142(1): 1-9.
- Lazic R, Gabric N (2007) Intravitreally administered bevacizumab (Avastin) in minimally classic and occult choroidal neovascularization secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 245(1): 68-73.
- Algvere PV, Steén B, Seregard S, Kvanta A (2008) A prospective study on intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration of different durations. Acta Ophthalmol 86(5): 482-489.
- Ehrlich R, Weinberger D, Priel E, Axer-Siegel R (2008) Outcome of bevacizumab (Avastin) injection in patients with age-related macular degeneration and low visual acuity. Retina 28(9): 1302-1307.
- Costagliola C, Romano M, Corte MD, Perrotta, Menzione M, Rinaldi M, et al. (2009) Intravitreal bevacizumab for treatment-naive patients with subfoveal occult choroidal neovascularization secondary to age-related macular degeneration a 12-month follow-up study. Retina 29(9): 1227- 1234.
- Landa G, Amde W, Doshi V, Ali A, McGevna L, et al. (2009) Comparative study of intravitreal bevacizumab Avastin) versus ranibizumab (Lucentis) in the treatment of neovascular age-related macular degeneration. Ophthalmologica 223(6): 370-375.
- Stepien KE, Rosenfeld PJ, Puliafito CA, Feuer W, Shi W, et al. (2009) Comparison of intravitreal bevacizumab followed by ranibizumab for the treatment of neovascular age-related macular degeneration. Retina 29(8): 1067-1073.
- Subramanian ML, Ness S, Abedi G, Ahmed E, Daly M, et al. (2009) Bevacizumab vs ranibizumab for age-related macular degeneration early results of a prospective double-masked, randomized clinical trial. Am J Ophthalmol 148(6): 875-882.
- Fong DS, Custis P, Howes J, Hsu JW (2010) Intravitreal bevacizumab and ranibizumab for age-related macular degeneration. A multicenter, retrospective study. Ophthalmology 117(2): 298-302.
- Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, Pan American Collaborative Retina Group, et al. (PACORES) (2008) Twelvemonth safety of intravitreal injections of bevacizumab (Avastin) results of the pan-american collaborative retina study group (PACORES). Graefes Arch Clin Exp Ophthalmol 246(1): 81-87.
- Wong LJ, Desai RU, Jain A, Feliciano D, Moshfeghi DM, et al. (2008) Surveillance for potential adverse events associated with the use of intravitreal bevacizumab for retinal and choroidal vascular disease. Retina 28(8): 1151-1158.
- Raftery J, Clegg A, Jones J, Seng Chuen Tan, Andrew Lotery (2007) Ranibizumab (Lucentis) versus bevacizumab (Avastin) modelling cost effectiveness. Br J Ophthalmol 91(9): 1244-1246.
- Cruess AF, Zlateva G, Xu X, Soubrane G, Pauleikhoff D, et al. (2008) Economic burden of bilateral neovascular age-related macular degeneration multi-country observational study. Pharmacoeconomics 26(1): 57-73.
- Smiddy WE (2009) Economic implications of current age-related macular degeneration treatments. Ophthalmology 116(3): 481-487.
- Baba T, Kubota-Taniai M, Kitahashi M, Kyoko Okada, Yoshinori Mitamura, et al. (2010) Two-year comparison of photodynamic therapy and intravitreal bevacizumab for treatment of myopic choroidal neovascularization. Br J Ophthalmol 94: 864-870.
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, et al. (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355(14): 1432-1444.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, et al. (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355(14):1419-1431.
- CATT Research Group, Martin DF, Maguire MG, et al. (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 364(20): 1897-1908.
- IVAN study.
- VIBERA study.
- LUCAS study.
- GEFAL study.
- MANTA study.
- Biswas P, Sengupta S, Choudhary R, Home S, Paul A, et al. (2011) Comparative role of intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age-related macular degeneration. Indian J Ophthalmol 59(3): 191-196.
- Lindblom B, Andersson T (1998) The Prognosis of Idiopathic Choroidal Neovascularization in Persons Younger Than 50 Years of Age. Ophthalmology 105(10): 1816-1820.
- Quaranta M, Mauget-Faysse M, Coscas G (2002) Exudative idiopathic polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Am J Ophthalmol 134(2): 277-280.
- Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, et al. (2008) 1-Year outcomes of photodynamic therapy in agerelated macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 115(1): 141-146.
- Silva RM, Figueira J, Cachulo ML, Duarte L, Faria de Abreu JR, et al. (2005) Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol 243(10): 973-979.
- Abouammoh M, Sharma S (2011) Ranibizumab versus bevacizumab for the treatment of neovascular age-related macular degeneration. Curr Opin Ophthalmol 22(3): 152-158.
- Subramanian ML, Abedi G, Ness S, Ahmed E, Fenberg M, et al. (2010) Bevacizumab vs ranibizumab for age-related macular degeneration 1-year outcomes of a prospective, double-masked randomised clinical trial. Eye (Lond) 24(11): 1708-1715.
- Dafer RM, Schneck M, Friberg TR, Jay WM (2007) Intravitreal Ranibizumab and Bevacizumab A Review of Risk. Semin Ophthalmol 22(3): 201-204.
- Kakinoki M, Sawada O, Sawada T, Kawamura H, Ohji M (2009) Comparison of macular thickness between cirrus HD-OCT and stratus OCT. Ophthalmic Surg Lasers Imaging 40(2): 135-140.
- Leung CK, Cheung CY, Weinreb RN, Lee G, Lin D, et al. (2008) Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 49(11): 4893-4897.
- Moon SW, Kim ES, Kim YG, Seung Young Yoo, Hyung Woo Kwak (2009) The comparison of macular thickness measurements and repeatabilities between time domain and spectral domain OCT. J Korean Ophthalmol Soc 50(7): 1050-1059.
© 2018 Jae Ryong Han. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






