- Submissions

Full Text
Modern Research in Dentistry
Hydroxyapatites as Bone Grafts and Coating Materials in Dental Science
Sohee Kang1, Hun Kim2, Yangho Lee2 and Inn-Kyu Kang2,3*
1Department of Dentistry, Korea
1Jeil Medical Corporation, Korea
1Department of Polymer Science and Engineering, Korea
*Corresponding author: Inn-Kyu Kang, Jeil Medical Corporation, Department of Polymer Science and Engineering, Daegu 41566, Korea
Submission: October 11, 2022;Published: October 20, 2022

ISSN:2637-7764Volume7 Issue4
Abstract
Hydroxyapatite (HA) is the main constituent of bones and teeth. Therefore, HA is widely used for artificial bone grafting, which is often used to replace the lost bones or in reconstructing alveolar bones before dental implantation. This study discusses the HA applications in dentistry. Once broken, the bones, comprising HA and collagen, regenerate over time. However, if enamel, which primarily comprises HA, is severely damaged, then it does not regenerate over time. This is because the enamel is devoid of collagen and nerves. Therefore, it is common to remove damaged teeth and transplant titanium implants if the tooth is considerably damaged. At this time, if the alveolar bone is reduced, a bone transplant is performed first. HA is a major material for artificial bone transplantation. It is also coated on the surface of dental implants to enhance tissue compatibility. To deposit HA on metal substrate surfaces, various methods, e.g., plasma thermal spraying, have been reported. Recently, research has been conducted to enhance the biological functionality by synthesizing nano-HA into various forms to deposit HA on the surface of implants or by introducing biomolecules on the surface of HA.
Keywords:Hydroxyapatite; Bone grafts; Hydroxyapatite deposition; Tissue compatibility; Implants
Introduction
In the event of trauma, birth defects, or disease, the damaged hard tissue must be reconstructed. Numerous studies of biomaterials have shown that calcium phosphate has been used to rebuild hard tissue for over 60 years. Hydroxyapatite (HA) is an inorganic mineral with a typical apatite lattice structure, such as Ca10(PO4)6(OH)2. Pure HA contains 39.68 wt% calcium and 18 wt% phosphorus, resulting in a Ca/P ratio of 1.67moles [1]. Bone contains 70% HA and 22% collagen, while tooth enamel contains 97% HA and dentin contains 69% HA and 20% organic matter [2]. Bone is a living tissue, composed of collagen, protein, and the mineral calcium phosphate, and has strong but flexible properties. Bones are constantly remodeled and regenerated throughout life [3]. Bone tissue does not remain the same and old tissue breaks down and new tissue is created. When a bone is broken, bone cells flock to the broken area and begin to regenerate the tissue. On the other hand, dental hard tissue has unique properties that are different from bone tissue. In teeth, living cells are inside the organ and are surrounded by calcified minerals. They are made up of four different types of tissue: enamel, dentin, cementum, and pulp. Dimensions are the innermost part of a tooth. It contains blood vessels, nerves and connective tissue. The pulp is surrounded by dentin covered with enamel. The enamel does not contain any nerves; it can be remineralized but cannot be regenerated or repaired in the event of serious damage.
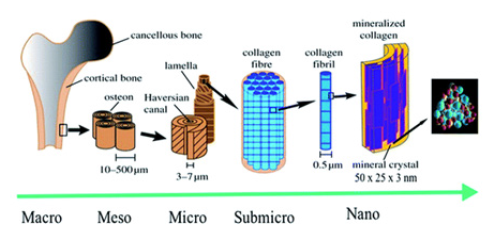
Bones have hierarchical structures similar to other biological structures, such as the intestines and muscles [4]. The lowest stage of this hierarchy is the uniform and highly ordered arrangement of HA nanocrystals in the collagen fibers. Depending on the characteristics and shape of the bones, nanocrystals are arranged in an orderly fashion by collagen fibers or layers. The HA nanocrystal is placed such that the c-axis of the HA nanocrystal resides along the fibril axis (Figure 1) [5].
Figure 1: Hierarchical structure of bone at various size scales. The microstructure of the cortical bone consists of osteons with Haversian canals and lamellae, whereas at the nanoscale, the structural units are collagen fibers composed of bundles of mineralized collagen fibrils (This figure was modified from the work of Zhang et al. [4] and Rho et al. [6]).
HA nanocrystals and nanoparticles have been widely used for the surface modification of dental implants [6,7]. HA powder coated at room temperature on the surface of the implant not only promotes adhesion of osteoblasts but also increases calcium deposition compared to that of traditional HA coatings [8]. HA is applied to various biomedical fields, such as drug release control support and bone tissue engineering materials [9]. Although synthetic HA and natural HA differ in physical microstructure, crystal size, and porosity, the chemical similarities to bone suggest that both synthetic and natural HA are bone conductive. Research and development of HA-based bioceramics is underway. In other words, strontium-HA, magnesium-HA, and silicone-HA are used to enhance the mechanical and biological properties in bone tissue engineering applications [10]. The mechanical strength of HA ceramics is low and is primarily restricted to implants under load. To overcome this limitation, HA, which improves sinterability and compactness as the surface area increases, was developed [11]. HA is a major enhancer in vertebrate hard tissue because it is strongly embedded in the collagen matrix of hard tissue in the form of nanoparticles. The use of HA as a reinforcing material has not been extensively studied. Suchanek et al. [12] reported that HA compounds could be reinforced with HA whiskers to increase mechanical properties without affecting biocompatibility. They used a 30% HA whisker to successfully improve fracture toughness by 40% compared to unreinforced HA. HA-metal composites are more suitable for loadbearing applications than HA-polymer composites due to their reliable mechanical properties [13]. Several HA-metal composites have been proposed as bone substitutes, such as HA-Ti, Ti-6Al-4VHA and HA-Mg. Research on HA-Ti composites is extensive because of their lower density, better corrosion and wear resistance, and lower Young’s modulus than other biometal implants. However, the thermochemical stability of HA and Ti has become an important issue in the study of HA-Ti composites [14]. Wataria et al. [15] reported that significant improvements have been achieved in the mechanical properties and osteogenesis of HA-Ti composites manufactured using the spark plasma sintering method. Although the mechanical strength of HA-Ti composites is comparable to that of bone, their long-term reliability is questionable due to their low interfacial bonding. Ceramic-ceramic composites also appear to be a better choice in terms of mechanical durability and chemical inertness in complex physiological environments.
In this review, we introduce the synthesis method of HA and explore the HA bone grafts, which are frequently used in dental implantation. We also investigated HA coating, which is used to enhance the bioactivity of dental implants. Finally, we discuss the role of HA in dentistry and future research directions.
HA Synthesis
Synthetic HA can be prepared using a variety of techniquesdry, wet, and high-temperature methods [16]. These methods generate different sizes and morphologies, and also yield calcium phosphate, a different crystal, along with the pure HA crystal. Consequently, the properties of HA significantly affect bioactivity and mechanical and biological properties. According to Cox et al. [17], since these properties determine the biomedical application of HA, it is interesting to develop synthetic methods to control the morphology, crystallinity, size, and chemical composition of HA. Studies of each synthesis method have been reviewed to determine the differences and complexities of each method in the synthesis of HA.
Dry method
HA synthesis by drying method can be classified into two types: solid state and mechanochemical method. In the dry method, HA was synthesized by mixing precursor chemicals (calcium and phosphate) in dry form. Sadat-Shojai et al. [16] reported that most drying methods do not require precise and controlled conditions. This drying method is suitable for mass production of powders. A solid-state reaction is defined as a reaction that decomposes by heating a mixed solid reactant to produce new solids and gases [18]. The solid-state method is considered a simple method to obtain HA by milling and calcifying a chemical system containing calcium and phosphate. Mechanochemical techniques use compression, shear or friction to induce chemical transformation through grinding and milling [19]. Mechanochemical techniques typically use ball or planetary milling at a specific speed or frequency. High impact compression [20] and increased local temperature [21] during mechanochemical reactions contribute to the chemical reaction and intensify the diffusion process [22].
Wet method
HA wet methods refer to the use of aqueous solutions while synthesizing HA. Wet methods commonly include chemical precipitation, hydrolysis, and hydrothermal methods. Wet methods can control the morphology and the average size of the powder. In addition to these advantages, wet methods also have some disadvantages, for HA exhibits low crystallinity owing to low processing temperatures [16]. Chemical precipitation is one of the most extensively researched technique used to synthesize HA. This method is widely used because it can synthesize large amounts of HA at a reasonable cost [23]. Chemical precipitation methods typically involve several processes. First, calcium hydroxide or calcium nitrate is used as a calcium source, and then orthophosphate or diammonium hydrogen phosphate is mixed as a phosphate source to match the mole ratio of HA. Subsequently, the mixture is adjusted to a specific pH in the alkali range, and the temperature is varied from room temperature to the boiling point of water [24-26]. Next, the solution is stirred for aging, washed, filtered, dried, and crushed into the powder form [17]. The hydrothermal method can be defined as a method that reacts to an aqueous medium above ambient pressure and temperature during synthesis [27]. The hydrothermal process occurs in environments with high temperatures and pressures, such as inside autoclaves and pressure vessels. Hydrolysis is one of the least commonly used wet methods for the synthesis of HA. Hydrolysis can be defined as the ionization of water that causes the diffusion of hydrogen and hydroxide ions [28]. Thus, the hydrolysis of calcium phosphate can form non-stoichiometric HA.
High-temperature method
HA can be synthesized using high-temperature methods that break down materials at high temperatures. These high-temperature methods include a combustion method and a pyrolysis method. However, combustion and pyrolysis methods are rarely used in the synthesis of HA. This is due to poor control over process parameters and secondary aggregate production [29]. Combustion technology uses a fast exothermic and self-sustaining redox reaction between an oxidant and an organic fuel in the water phase [29]. The reaction is initiated by heating the mixture at a lower temperature before a sudden increase in temperature. The final step in this process is rapid cooling, which induces nucleation and prevents further grain growth. Pyrolysis techniques involve spraying a precursor material into a hot region of an electric furnace [30].
HA as Bone Grafts in Dental Science
One successful way to reconstruct missing attachments due to deep bone defects is to implant bone replacement materials [31]. Bone replacement transplants consist of autogenous allografts, xenografts, and alloplasts. Alloplastic materials include synthetic, inorganic, or bioactive bone replacements. They are widely used because they can be easily procured [32]. In addition, calcium phosphate ceramics are widely used to treat intraperitoneal defects as bone graft replacements. Significant clinical results were obtained by a study that used the aforementioned materials [33].
These observations indicate that the performance of a porous bone graft replacement depends on the pore structure as a function of interconnection and pore volume [34]. Haas et al. [35] investigated the value of non-resorbable porous HA as an implant in a single-lift procedure using an adult female sheep. As a result, all implants were bone fused to the local host bone, and new bone formation was observed in the triangular region consisting of the implant surface, local buccal antral wall, and submucosal connective tissue. Kanaya et al. [36] reported that HA could stimulate the differentiation of periodontal cells mediated by mechanosensitive signals and expression of bone morphogenetic proteins 2 (BMP-2). Furthermore, Yang et al. [37] found that HA could be used as a coating material for silk scaffolds through animal experiments. Therefore, HA-coated silk scaffolds could potentially be a good biomaterial for regenerating periodontal tissue. Along with the work mentioned above, there are several studies in the existing literature that highlight the effect of HA on different cells in the periodontium. Recently, Vullo et al. [38] successfully applied HA with crystals measuring 70-100 nm as a grafting material for regenerative periodontal therapy in dogs. Their study demonstrated that HA represents a valid bone conductive and bone-induced transplant product that confirmed the regenerability of periodontal therapy in dogs. Chitsazi et al. [39] compared the efficacy of HA with self-generated bone grafts in the treatment of intraperitoneal bone defects. The result was an effective treatment for two- and three-wall bone defects. The efficacy of HA with enamel matrix derivatives in treating intravenous defects was compared by Machot et al. [40]. Some synthetic bone grafts are made from calcium carbonate, which can be completely reduced in a short period of time and start to reduce usage as the bones break easily. The combination of tricalcium phosphate and HA affects the bone conduction and resorbability. Prathap et al. [41] used HA in an interactive grade II treatment involving the mandibular first molar.
Applying HA, both with and without collagen membranes, significantly reduced the depth of the horizontal and vertical probes and increased the level of clinical attachment. Although not statistically significant, the use of collagen membranes resulted in superior results compared to those of the area treated with bone graft alone. Clinical and radiographic results were evaluated by Singh et al. [42] in HA alone and in combination with bio-absorbable collagen membranes in the treatment of intraperitoneal defects. It has been concluded that the HA produced by the combination therapy was much greater just by opening the fragment removal procedure. Kamboj et al. [43] evaluated the effectiveness of HA bone grafts in treating skeletal defects in the human body, and they suggested that HA had a significant effect on pocket depth reduction and on statistically significant gains at the depth and attachment levels of elliptical lesions. Bansal et al. [44] revealed that applying HA can improve clinical and radiographic parameters in the treatment of intraperitoneal skeletal defects. Kasaj et al. [45] compared the clinical results of intrabony periodontal defects after treatment with HA paste and open flap fragment decomposition. After treating intrabony periodontal defects with the HA paste, a significant improvement was reported in the clinical outcomes over open flap destruction. Elgendy et al. [46] compared the clinical and radiographic outcomes of HA with and without Platelet-Rich Fibrin (PRF) membranes in the treatment of intraperitoneal bone defects and concluded that HA is an appropriate bone substitute for the treatment of periodontal defects. Rahman et al. [47] used PRF by combining HA and collagen to treat surrounding lesions. They reported that this novel technique was an effective way to induce faster periodic healing in large periapical lesions.
In conclusion, HA can be considered an appropriate alternative to autogenous bone grafts for periodontal tissue regeneration. The use of this type of material has been on the rise in recent years because it has several benefits, including minimizing patient disease, biological fitness, and lack of toxicity. When HA is combined with other active particles or biological intermediates, the application of HA alone can further stimulate the periodontal tissue regeneration [38]. More randomized controlled clinical studies must be conducted on various applications of biomaterials in the treatment of periodontal defects. In the experimental studies on the adult female sheep, the value of non-resorbable porous HA as a grafting material in a single-stage sinus-lift procedure was investigated. As a result, all implants were osseointegrated into the host bone. New bone formation has been traced to triangular regions bounded by implant surfaces, local buccal walls, and submucosal connective tissue around all implants. With regard to the bone graft contact, there was no significant difference between HA and the autogenous bone [35].
HA Coating on Metal or Ceramic Implants
HA is one of the widely used calcium phosphate coatings for titanium implants [48]. The widespread use of HA in biomedical fields can be attributed to its non-toxic and non-inflammatory properties, and excellent osteogenesis, osseointegration, bone induction, biodegradability, and corrosion resiliency [49,50]. Unfortunately, HA is not only vulnerable but also has low fracture strength and fatigue. These drawbacks limit the application of clinical load bearings [51]. To overcome these shortcomings, HA has been primarily used to cover metal surfaces. HA and metal substrate-based implants combine the biological properties of HA with the superior mechanical properties of the metal [52]. HA deposition on metal substrate surfaces is performed in various ways, including plasma deposition [53], sol-gel method [54], vapor deposition [55], ion-beam assisted deposition [56], pulsed laser deposition [57], RF magnetron sputtering [58], dip coating [59], biomimetic coating [60], dynamic mixing [61], electrophoretic [62], and electrochemical deposition [63]. Among these HA deposition methods, the plasma spraying method has been studied and used for the longest time [64]. However, this technique has serious limitations because the high temperatures and rapid cooling associated with this process scatter various chemical phases and reduce the crystallinity of HA.
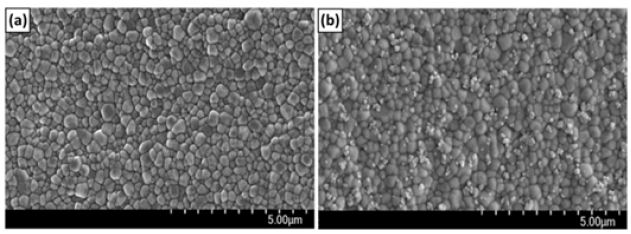
For physical coating methods, HA deformation is likely to occur after stacking because of the low fracture strength of HA. To overcome this, Park et al. [48] conducted a study to introduce nano-sized HA as a chemical covalent bond to titanium implant surfaces. As the results, the nHA-conjugated Ti disk implants have shown enhanced adhesion, proliferation and cytotoxicity of MC3T3-E1 cells in comparison to pristine Ti implants. Kim et al. [65] introduced nHA and type I collagen to the zirconia surface by chemical covalent bonding to increase bioactivity and ensure safety in the body. They confirmed the chemical reaction of the surface by X-ray photoelectron spectroscopy (XPS), field emission scanning electron microscopy (Figure 2). As a result, nHA-immobilized zirconia (ZrO2-HA) and nHA- and type I collagen-immobilized zirconia (ZrO2-HA-Col), which showed high bioactivity in in vitro osteoblast experiments.
Figure 2: Scanning electron microscopy images of ZrO2 disk (a) and ZrO2 -HA disk (b). It can be seen that the zirconia disk (a) obtained by injection molding is composed of granules with a diameter of 200 to 500nm. On the other hand, after the nHA reaction, single nHA and nHA clusters were bound to the zirconia surface in a dispersed state [65].
Plasma spray deposition produces an amorphous calcium phosphate derivative with a mixture of nano- and micrograin sizes on the metal surface. Nobre et al. [66] conducted a field pilot study to evaluate the effectiveness of three HA-based solutions and their interactions with different dental material surfaces under oral conditions. They concluded that HA could adhere not only to enamel but also to artificial tooth surfaces such as titanium, ceramics, and Polymethylmethacrylate (PMMA) under oral conditions. The experimental results demonstrated that the obtained pellicle acted as a bridge between HA and the surface of the material [67,68].
Sealing non-cavitated carious lesions in pits and cracks leads to sealant failure due to incomplete sealing. Therefore, the use of remineralizing agents, such as nanoparticles, has been proposed. Memarpour et al. [69] investigated the ability of HA to remineralize enamel and its effect on sealant microleakage and shear bond strength (SBS) [69]. As a result, HA reinforces SBS, thereby remineralizing the enamel when the nanoparticle content is high. They suggested that SBS is related to the HA concentration. HA 0.15% yielded SBS (16.8±2.7) greater than 0.03% concentration (14.2±2.1). However, the effects of microleakage were not significant. Bordea et al. [70] analyzed 32 studies using keywords from HA and dentistry in two databases (Scoups and PubMed). The main applications of HA in implantation are its coating material on titanium, and its osteointegration, inflammatory response, and antibacterial activity. In terms of tissue engineering, significant attention has been paid to surgery and periodontal pathology. In addition, in esthetic and prevention, ivory perceptual hypersensitivity treatment was the primary focus, and in remineralization prevention, the content was mainly focused on the possibility of ivory perceptual hypersensitivity treatment or remineralization [71]. Recently, research has been conducted to enhance the affinity with bone cells through surface reformation of nano HA [72]. Haider et al. [67] introduced BMP through a chemical bond with the HA surface (Figure 3). This was mixed with biodegradable polymers to fabricate a composite nanofiber scaffold. Upon examining their interactions with osteoblasts, the cells grew and differentiated more rapidly in HA-containing scaffolds. These results suggest that surface remodeling of HA may increase the suitability of the dental tissue. Haider et al. [68] prepared biocomposites with PLGA using nano-spherical HA (sHA) and nano-rod HA (nHA) and investigated their effect on the biological activity of osteoblasts [73]. As seen in Figure 3, sHA has more negatively charged PO43- ions on the outer surface, in contrast to nHA, which has an overall positive charge on the outer surface due to the presence of Ca2+ ions; thus, nHA offers more chances for osteoblastic cell adhesion as osteoblastic cells are predominantly negatively charged [74]. In their interaction experiments with osteoblasts and biocomposites containing different morphologies of HA, nHA promoted cell growth and differentiation significantly faster than sHA.
Figure 3: Schematic representation of grafting of (a) BMP-2, and (b) insulin on the surface of nHA (This figure was modified from the work of Haider et al. [67] and [68]).

Conclusion
HA is a promising material for various dental treatments. The HA implant demonstrated better performance than other materials used as titanium implant coatings, and it also exhibits remarkable properties as a sinus lifting material. As a tissue engineering material, HA presents improved bone regeneration properties compared to that of the autonomous bone transplantation. Combined with other scaffolding, it demonstrated remarkable performance in surgery and periodontal intervention. HA has also been studied as an adjuvant to bleach treatment, as well as an element of dental hypersensitivity and remineralizing therapy, making it suitable for esthetics and preventive medicine. HA is a relatively new material, and its application in dentistry should be further examined. Its physical, chemical, and mechanical properties make it one of the most promising materials in modern dentistry. Finally, it may be possible to combine physiologically active substances such as collagen [75] and cell growth factors [67,68] on the surface of HA and use them as bone grafting materials. In addition, by combining drugs [76] with HA on the surface of metal or ceramic implants, the function of a drug carrier that could not be represented by HA alone could be expected (Figure 4).
Figure 4:Schematic of the osteoblastic cells’ response to different morphological forms of HA nanoparticles [73

Funding
This work was supported by the 2022 Yeungnam University research grant.
References
- Oguchi H, Ishikawa K, Mizoue K, Seto K, Eguchi G (1995) Long-term histological evaluation of hydroxyapatite ceramics in humans. Biomaterials 16(1): 33-38.
- Pepla E, Besherat LK, Palaia G, Tenore G, Migliau G (20140 Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann Stomatol 5(3):108-114.
- Raggatt LJ, Partridg NC (2010) Cellular and molecular mechanisms of bone remodeling. J Biolog Chem 285(33): 25103-25108.
- Zhang Z, Zhang YW, Gao H (2010) On optimal hierarchy of load-bearing biological materials. Proc Biol Sci 278(1705): 519-525.
- Haider A, Haider S, Han SS, Kang IK (2017) Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: a review. RSC Adv 7(13): 7442-7458.
- Rho JYL, Spearing K, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20(2): 92-102.
- Breding K, Jimbo R, Hayashi M, Mustafa K, Anderson M (2014) The effect of hydroxyapatite nanocrystals on osseointegration of titanium implants: An in vivo rabbit study. Int J Den 2014: 171305.
- Sato M, Sambito MA, Aslani A, Kalkhoran NM, Slamovich EB, et al. (2006) Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials 27(11): 2358-2369.
- Lai W, Chen C, Ren X, Lee IS, Jiang G, et al. (2016) Hydrothermal fabrication of porous hollow hydroxyapatite microspheres for a drug delivery system. Mater Sci Eng C 62: 166-172.
- Rasskazova LA, Zhuk IV, Korotchenko NM, Brichkov AS, Chen YW, et al. (2019) Synthesis of magnesium- and silicon-modified hydroxyapatites by microwave-assisted method. Scientific Reports 9: 14836.
- Bayani M, Torabi S, Shahnaz A, Pourali M (2017) Main properties of nanocrystalline hydroxyapatite as a bone graft material in treatment of periodontal defects. A review of literature. Biotech Biotechno Equip 31(2): 215-220.
- Suchanek W, Yashima M, Kakihana M, Yoshimura M (1997) Hydroxyapatite/hydroxyapatite-whisker composites without sintering additives: Mechanical properties and microstructural evolution. J Am Cer Soc 80(11): 2805-2813.
- Arifin A, Sulong AB, Muhamad N, Syarif J, Ramli MI (2014) Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater Design 55: 165-175.
- Mahmud NN, Sulong AB, Sharma B, Ameyama K (2021) Presintered titanium-hydroxyapatite composite fabricated via PIM route. Metals 11(2): 318-327.
- Watari F, Miyao R, Matsuno H, Uo M, Kawasaki T, et al. (2001) Mechanical properties and biocompatibility of titanium-hydroxyapatite implant materials prepared by spark plasma sintering method. Key Eng Mater 192-195: 445-448.
- Sadat-Shojai M, Khorasani MT, Dinpanah KE, Jamshidi A (2013) Synthesis methods for nano-sized hydroxyapatite with diverse structures. Acta Biomater 9(8): 7591-7621.
- Cox SC, Mallick KK, Walton RI (2014) Comparison of techniques for the synthesis of hydroxyapatite, Bioinspired, Biomim. Nanobiomaterials 4(1): 37-47.
- Rahaman M, Rahaman MN (2006) Ceramic Processing, Taylor & Francis, UK.
- Achar TK, Bose A, Mal P (2017) Mechanochemical synthesis of small organic molecules. J Org Chem 13: 1907-1931.
- Shen TD, Koch CC, Mccormick TL, Nemanich RJ, Huang JY, et al. (1995) The structure and property characteristics of amorphous/nanocrystalline silicon produced by ball milling. J Mater Res 10: 139-148.
- Schwarz RB (1996) Scr Mater 34: 1-4.
- Yeon KC, Wang J, Ng SC (2001) Mechanochemical synthesis of nanocrystalline hydroxyapatite from CaO and CaHPO4. Biomaterials 22(2): 2705-2712.
- Yelten YA, Yilmaz S (2018) Wet chemical precipitation synthesis of hydroxyapatite (HA) powders. Ceram Int 44(8): 9703-9710.
- Catros S, Guillemot F, Lebraud E, Chanseau C, Perez S, et al. (2010) Physico-chemical and biological properties of a nano-hydroxyapatite powder synthesized at room temperature. IRBM 31(4): 226-233.
- Gomes DS, Santos AMC, Neves GA, Menezes RR (2019) A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 65: 282-302.
- Kwak S, Haider A, Gupta KC, Kim S, Kang IK (2016) Micro/Nano multilayered scaffolds of PLGA and collagen by alternately electrospinning for bone tissue engineering. Nanoscale Research Letters 11(1): 323.
- Zhang Tao, Xiao X (2020) Hydrothermal synthesis of hydroxyapatite assisted by gemini cationic surfactant. J Nanomater.
- Sinitsyna OV, Veresov AG, Kovaleva ES, Kolen’ko YV, Putlyaev VI, et al. (2005) Synthesis of hydroxyapatite by hydrolysis of α-Ca3 (PO4)2. Russian Chemical Bulletin 54: 79-86.
- Kavitha M, Subramanian R, Narayanan R, Udhayabanu V (2014) Solution combustion synthesis and characterization of strontium substituted hydroxyapatite nanocrystals. Powder Technol 253: 129-137.
- Widiyastuti W, Setiawan A, Winardi S, Nurtono T, Setyawan H (2014) Particle formation of hydroxyapatite precursor containing two components in a spray pyrolysis process. Front Chem Sci Eng 8: 104-113.
- Scabbia A, Trombelli LA (2004) Comparative study of the use of HA/collagen/chondroitin sulphate biomaterial (Biostite) and a bovine-derived HA xenograft (Bio-Oss) in the treatment of deep intra-osseous defects. J Clin Periodontol 31(5): 348–355.
- Aichelmann-Reidy ME, Yukna RA (1998) Bone replacement grafts. The bone substitutes. Dent Clin North Am 42(3): 491- 503.
- Saffar JL, Colombier ML, Detienville R (1990) Bone formation in tricalcium phosphate-filled periodontal intrabony lesions. Histological observations in humans. J Periodontol 61(4): 209-216.
- Kenney EB, Lekovic V, Han T, Carranza FA, Dimitrijevic B (1985) The use of a porous hydroxylapatite implant in periodontal defects. I. Clinical results after six months. J Periodontol 56(2): 82-88.
- Haas R, Baron M, Donath K, Zechner W, Watzek G (2002) Porous hydroxyapatite for grafting the mmaxillary sinus: A comparative histomorphometric study in sheep. Int J Oral Maxillofac Implants 17(3): 337-346.
- Kanaya S, Nemoto E, Yukihiko S, Shimauchi H (2013) Calcium-mediated increased expression of fibroblast growth factor-2 acts through NF-kappa B and PGE2/EP4 receptor signaling pathways in cementoblasts. Bone 56(2): 398-405.
- Yang C, Lee JS, Jung UW, Seo YK, Park JK, et al. (2013) Periodontal regeneration with nano-hyroxyapatite-coated silk scaffolds in dogs. J Periodontal Implant Sci 43(6): 315-322.
- Vullo C, Meligrana M, Rossi G, Tambella AM, Dini F, et al. (2015) Use of nanohydroxyapatite in regenerative therapy in dogs affected by periodontopathy. Ann Clin Lab Res 3(2): 18.
- Chitsazi MT, Shirmohammadi A, Faramarzie M, Pourabbas R, Rostamzadeh AN (2011) A clinical comparison of nano-crystalline hydroxyapatite (Ostim) and autogenous bone graft in the treatment of periodontal intrabony defects. Med Oral Patol Oral Cir Bucal 16(3): e448-e453.
- Machot EA, Hoffmann T, Lorenz K, Khalili I, Noack B (2014) Clinical outcomes after treatment of priodontal intrabony defects with nanocrystalline hydroxyapatite (Ostim) or enamel matrix derivatives (Emdogain): A randomized controlled clinical trial. BioMed Res Int 2014: 786353.
- Prathap S, Hegde S, Kashyap R, Prathap MS, Arunkumar MS (2013) Clinical evaluation of porous hydroxyapatite bone graft (Periobone G) with and without collagen membrane (Periocol) in the treatment of bilateral grade II furcation defects in mandibular first permanent molars. J Indian Soc Periodontol 17(2): 228-234.
- Singh VP, Nayak DG, Uppoor AS, Shah D (2012) Clinical and radiographic evaluation of nano-crystalline hydroxyapatite bone graft (Sybograf) in combination with bioresorbable collagen membrane (Periocol) in periodontal intrabony defects. Dent Res J 9(1): 60-67.
- Kamboj M, Harinder R, Gupta A (2016) Comparative evaluation of the efficacy of synthetic nanocrystalline hydroxyapatite bone graft (Ostim®) and synthetic microcrystalline hydroxyapatite bone graft (Osteogen®) in the treatment of human periodontal intrabony defects: A clinical and denta scan study. J Indian Soc Periodontol 20(4): 423-428.
- Bansal M, Kaushik M, Khattak BP, Sharmaet A (2014) Comparison of nanocrystalline hydroxyapatite and synthetic resorbable hydroxyapatite graft in the treatment of intrabony defects: A clinical and radiographic study. J Indian Soc Periodontol 18(2): 213-219.
- Kasaj A, Rohrig B, Zafiropoulos GG, Zafiropoulos GG, Willershausenet B (2008) Clinical evaluation of nanocrystalline hydroxyapatite paste in the treatment of human periodontal bony defects - A randomized controlled clinical trial: 6-month results. J Periodontol 79(3): 394-400.
- Elgendy EA, Shady TEA (2015) Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet-rich fibrin membrane in the treatment of periodontal intrabony defects. J Indian Soc Periodontol 19(1): 61-65.
- Rahman H, Chandra A, Aziz A, Ramesh B (2015) Platelet rich fibrin and nanocrystalline hydroxyapatite with collagen combination in treatment of periapical lesion: A novel clinical approach. British J Med Med Res 5(2): 275-282.
- Park SJ, Kim BS, Gupta KC, Lee DY, Kang IK (2018) Hydroxyapatite nanorod-modified sand blasted titanium disk for endosseous dental implant applications. Tissue Eng Regen Med 15(5): 601-614.
- Hench LL (1991) Bioceramics: from concept to clinic. J Am Ceram Soc 74(7): 1487-1510.
- Bramowicz M, Braic L, Azem FA, Kulesza S, Birlik I, et al. (2016) Mechanical properties and fractal analysis of the surface texture of sputtered hydroxyapatite coatings. Appl Surf Sci 379: 338-346.
- Gopi D, Shinyjoy E, Karthika A, Nithiya S, Kavitha L, et al. (2015) Single walled carbon nanotubes reinforced mineralized hydroxyapatite composite coatings on titanium for improved biocompatible implant applications. RSC Adv 5(46): 36766-36778.
- Vladescu A, Padmanabhan SC, Ak Azem F, Braic M, Titorencu I, et al. (2016) Mechanical properties and biocompatibility of the sputtered Ti doped hydroxyapatite. J Mech Behav Biomed Mater 63: 314-325.
- Coyle TW, Garcia E, Zhang Z, Gan L (2007) Plasma spray deposition of hydroxyapatite coatings from sol precusors. Mater Sci Forum 539-543: 1128-1133.
- Bezzi G, Celotti G, Landi E, La Torretta TMG, Sopyan I, et al. (2003) A novel sol–gel technique for hydroxyapatite preparation. Mater Chem Phy 78(3): 816-824.
- Hou X, Choy KL, Leach SE (2007) Processing and in vitro behavior of hydroxyapatite coatings prepared by electrostatic spray assisted vapor deposition method. J Biomed Mater Res A 83(3): 683-691.
- Choi JM, Kim HE, Lee IS (2000) Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials 21(5): 469-473.
- Dinda GP, Shin J, Mazumder J (2009) Pulsed laser deposition of hydroxyapatite thin films on Ti–6Al–4V: Effect of heat treatment on structure and properties. Acta Biomater 5(5): 1821-1830.
- Prosolov KA, Popova KS, Belyavskaya OA, Rau JV, Gross KA, et al. (2017) RF magnetron-sputtered coatings deposited from biphasic calcium phosphate targets for biomedical implant applications. Bioact Mater 2(3): 170-176.
- Lacefield WR (1988) Hydroxyapatite Coating. Ann N Y Acad Sci 523: 72-80.
- Habibovic P, Barrere F, Blitterswijk CA, Groot K, Layrolle P (2004) Biomimetic hydroxyapatite coating on metal implants. J Am Cer Soc 85(3): 517-522.
- Yoshinari M, Ohtsuka Y, Dérand T (1994) Thin hydroxyapatite coating produced by the ion beam dynamic mixing method. Biomaterials 15(7): 529-535.
- Sorkhi L, Farrokhi-Rad M, Shahrabi T (2019) Electrophoretic deposition of hydroxyapatite- chitosan-titania on stainless steel 316 L. Surfaces 2(3): 458-467.
- Yuan Z, Jie HT, Ying-Chun PWW, Wang W, Tao W (2006) Electrochemical deposition of hydroxyapatite coatings on titanium. Trans Nonferrous Met Soc China 16(3): 633-637.
- Khor KA, Cheang P (1997) Plasma sprayed hydroxyapatite (HA) coatings produced with flame spheroidised powders. J Mater Proc Tech 63(1-3): 271-276.
- Kim H, Lee YH, Kim NK, Kang IK (2022) Bioactive surface of zirconia implant prepared by nano-hydroxyapatite and type I collagen. Coatings 12(9): 1335.
- Nobre CMG, Pütz N, Hannig M (2020) Adhesion of hydroxyapatite nanoparticles to dental materials under oral conditions. Scanning.
- Haider A, Kim S, Huh MW, Kang IK (2015) BMP-2 grafted nHA/PLGA hybrid nanofiber scaffold stimulates osteoblastic cells growth. BioMed Res Int 2015: 281909.
- Haider A, Gupta KC, Kang IK (2014) PLGA/nHA hybrid nanofiber scaffold as a nanocargo carrier of insulin for accelerating bone tissue regeneration. Nanoscale Res Lett 9(1): 314.
- Memarpour M, Shafiei F, Rafiee A, Soltani M, Dashti MH (2019) Effect of hydroxyapatite nanoparticles on enamel remineralization and estimation of fissure sealant bond strength to remineralized tooth surfaces: an in vitro BMC Oral Health 19(1): 92-104.
- Bordea IR, Candrea S, Alexescu G, Bran S, Băciuț M, et al. (2020) Nano-hydroxyapatite use in dentistry: a systematic review. Drug Metabo Rev 52(2): 319-332.
- Prabhakar AR, Manojkumar AJ, Basappa N (2013) In vitro remineralization of enamel subsurface lesions and assessment of dentine tubule occlusion from NaF dentifrices with and without calcium. J Indian Soc Pedod Prev Dent 31(1): 29-35.
- Meirelles L, Arvidsson A, Andersson M, Kjellin P, Albrektsson T, et al. (2008) Nano hydroxyapatite structures influence early bone formation. J Biomed Mater Res 87(2): 299-307.
- Haider A, Gupta KC, Kang IK (2014) Morphological effects of HA on the cell Compatibility of electrospun HA/PLGA composite nanofiber scaffolds. BioMed Res Int 2014: 308306.
- Kandori K, Oda S, Fukusumi M, Morisada Y (2009) Synthesis of positively charged calcium hydroxyapatite nanocrystals and their adsorption behavior of proteins. Colloids and Surf B Biointerfaces 73(1): 140-145.
- Xing ZC, Chang KW, Chun S, Kim S, Kang IK (2014) Immobilization of collagen on hydroxyapatite discs by covalent bonding and physical adsorption and their interaction with MC3T3-E1 osteoblasts. Tissue Eng Reg Med 11: 1-7.
- Haider A, Versace DL, Gupta KC, Kang IK (2016) Pamidronic acid-grafted nHA/PLGA hybrid nanofiber scaffolds suppress osteoclastic cell viability and enhance osteoblastic cell activity. J Mater Chem B 4(47): 7596-7604.
© 2022 Inn-Kyu Kang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






