- Submissions

Full Text
Modern Approaches in Drug Designing
4-((Bis (2-Chloroethyl) Amino) Methyl)-2H-Chromen- 2-Ones as Antifungal, Cytotoxic, Anti-tubercular and Antimicrobial Agents
Naik RJ1, Revankar HM1, Kulkarni MV1*, Pai KSR2 and Nayak PG2
1Department of Chemistry, Karnatak University, India
2Department of Pharmacology, Manipal University, India
*Corresponding author: MV Kulkarni, PG Department of Chemistry, Karnatak University, Dharwad- 580003, Karnataka, India
Submission: October 12, 2017; Published: December 11, 2017

ISSN : 2576-9170Volume1 Issue3
Abstract
4-((Bis(2-chloroethyl)amino)methyl)-2H-chromen-2-ones synthesised from 4-Bromomethyl coumarins were found to exhibit a broad spectrum antifungal activity with IC50 of 0.2μg/mL. They exhibited moderate activity against HeLa and MCF-7 cell lines. They exhibited promising activity against M.tuberculosis and bacterial strains. Cytotoxic studies revealed that all the screened compounds showed IC50 higher than 5μg/mL against V79 and HBL100.
Keywords: 2H-chromen-2-ones; Cell line screening; Anti-tubercular; Anti-microbial; Anti-fungal
Introduction
The concept of possessing an active and a carrier moiety as a pre-requisite for pharmacological activity has resulted in the use of cytostatically active nitrogen mustards, in combination with a carrier to produce a host of potential anti-cancer agents [1]. Coumarin is a group of naturally occurring lactones exhibiting a wide spectrum of biological activities like antibacterial [2], antifungal [3], antiviral [4], anti-tubercular [5], anti-malarial [6], anti-inflammatory [7], anticancer [8] and anti HIV [9]. Role of coumarins in cancer chemotherapy has been reviewed and clinically acceptable results have been observed with psoralens useful in the treatment of skin cancer [10]. Simple derivatives of coumarin like 7-hydroxy 4-methyl coumarin [11], osthole [12], nitrated 7-hydroxy coumarins and nitrogen mustards of corresponding Mannich bases [13] have shown promising activity in different forms of cancer in mice and cell line studies.
Allylic position in the pyran ring has been employed for the construction of anti-microbial triheterocycles [14], and nitrogen heterocycles [15]. Introduction of a basic side chain at the allylic position with respect to the biogenetically important C3-C4 double bond reduces the acidic character of coumarin antibiotics. In particular, presence of piperazine and imidazole moieties at the allylic position considerably enhances the antimicrobial activity [11]. Introduction of the above linkages with a variety of other substituents into the coumarin ring was employed as a strategy to design new anti-bacterial compounds [11].
In view of the above cited literature, introduction of bis (2-chloroethyl) amino moiety at the allylic position of coumarin ring was thought to be quite pertinent. Since bis (2-chloroethyl) amines are well known alkylating agents employed in cancer chemotherapy they were screened against HeLa and MCF-7 cell lines. Presence of basic side chain at the allylic position of coumarin ring was exploited for its anti-microbial activity.
Chemistry
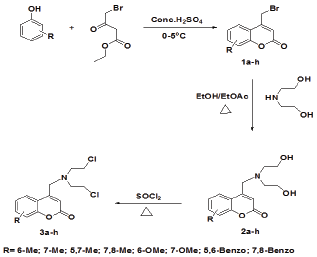
Ethyl 4-bromo-3-oxobutanoate obtained from the bromination of ethyl 3-oxobutanoate, was treated with substituted phenols under Pechmann cyclization conditions using neat sulphuric acid as the condensing agent. The reaction resulted in the formation of 4-(bromomethyl)-2H-chromen-2-ones 1a-h. The reactivity of these compounds has been explored in a cascaded manner via an allylic displacement followed by an SNi reaction using thionyl chloride. Initial problem in the condensation of diethanol amine was its viscous nature which leads to difference in the solubilities with 1a-h. However a mixture (1:1) of ethyl acetate and ethanol proved to be a successful medium. An equimolar quantity of compounds 1a-h and diethanol amine C were refluxed with 1:1 mixture of ethyl acetate and ethyl alcohol to get 4-{[bis (2-hydroxyethyl) amino] methyl}-2H-chromen-2-ones 2a-h. 4-{[bis (2-chloroethyl) amino] methyl}-2H-chromen-2-ones 3a-h were obtained by heating compounds 2a-h with thionyl chloride at 60-70 °C for 15 min. Intermediates 2a-h and title compounds 3a-h were purified by column chromatography and structures were supported by spectral data (Scheme 1).
Scheme 1: Synthesis of 3a-h via nucleophillic substitution and subsequent chlorination.
Results and Discussion
Formation of compound 2a was confirmed by spectral methods, In IR spectrum of 2a, a strong absorption band at 1710 cm-1 indicated the presence of lactone carbonyl group and broad band at 3387 cm-1 indicated the presence of hydroxyl groups. In 1H NMR spectrum two broad singlets at 2.00ppm and 3.22ppm integrating for one proton each indicated the presence of two hydroxyl groups, which was further confirmed by D2O exchange. Singlet appearing at 2.42ppm integrating for three protons indicated the presence of C6- methyl group. Two triplets appearing at 2.82 δ ppm and 3.70ppm integrating for eight protons attributed to the N-CH2 and HO-CH2 protons respectively. Singlet at 3.92ppm integrating for two protons was corresponded to C4- methylene protons. Singlet at 6.71ppm integrating for one proton indicated the presence of C3-proton. Doublet at 7.21ppm and 7.32ppm with coupling constant 9.0Hz and integrating for two protons attributed to C8 and C7 protons respectively. Singlet at 7.56ppm integrating for single proton indicated the presence of C5 proton. In mass spectrum, molecular ion peak was observed at m/z 278(M+1).
In IR spectrum of 3a, a strong absorption band at 1703 cm-1 indicated the presence of lactone carbonyl group. In 1H NMR spectrum singlet appearing at 2.34ppm integrating for three protons indicated the presence of C6- methyl group. Two triplets appearing at 2.97 δ ppm and 3.50ppm integrating for eight protons attributed to the N-2 and Cl-CH2 protons respectively. Singlet at 3.89ppm integrating for two protons was corresponded to C4- methylene protons. Singlet at 6.56ppm integrating for one proton indicated the presence of C3-proton. Doublet at 7.17ppm and 7.27ppm with coupling constant 9.0Hz and integrating for two protons attributed to C8 and C7 protons respectively. Singlet at 7.52ppm integrating for single proton indicated the presence of C5 proton. In 13C NMR spectrum, C6- methyl carbon appeared at 21.3ppm. The C4- methylene carbon observed at 57.0ppm. N-CH2 and Cl-CH2 carbons resonated at 42.0ppm and 56.3ppm respectively. The olefinic and aromatic carbons were observed at 114.9, 115.0, 117.2, 118.4, 124.5, 134.3, 152.1, 152.7ppm. Lactone carbonyl carbon resonated at 161.5ppm. In mass spectrum, molecular ion peak was observed at m/z 313.
Pharmacology
In present study electron donating substituents at C6 and C7 positions in the form of methyl and methoxy groups were chosen. The study was extended to disubstituted coumarins where in 5,7 and 7,8-dimethyl substituents were considered. To evaluate the extended planarity and stereo electronic effects, 5,6-benzo and 7,8-benzo coumarins have been selected.
Cell line activity against MCF-7 and HeLa
Table 1: Drug incubation with HeLa and MCF-7 cell lines by MTT assay and cytotoxic activities with V79 and HBL100 in μg/mL.
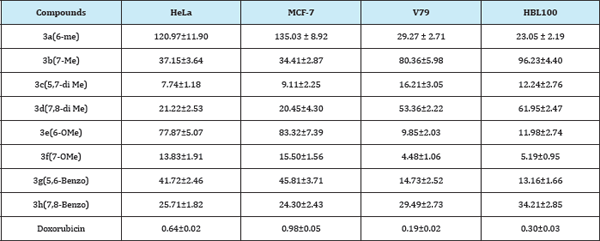
Compounds 3a-h was evaluated for their cell line activity against MCF-7 (human breast adenocarcinoma) and HeLa (human cervical tumor cells) using MTT cytotoxic assay. Structure activity relationship studies indicated that electron donating substituents at C7 position were effective than at C6 position. Amongst the two groups located at C7, methoxy group 3f was more effective than the methyl substituted derivative 3b, where in former exhibited lower IC50 values of 13.83μM/mL and 15.50 μM/mL against HeLa and MCF-7 cell lines respectively. 5,7-dimethyl substituted compound 3c was found to be the most potent in the series exhibited lowest IC50 values of 7.74μM/mL and 9.11μM/mL against HeLa and MCF- 7 cell lines respectively. Introduction of the benzo substituent showed signs of improvement in the activity when it was observed that the 7,8-benzo derivative 3h with IC50 values of 25.70μM/mL and 24.30μM/mL against HeLa and MCF-7 cell lines was better than 7-methyl derivative 3b. However the same group at 5,6-positions leading to 3g resulted in considerable loss of activity (Table 1).
Cytotoxic Study
Cytotoxic study with normal cells (V79 and HBL100) showed IC50 more than 5μg/mL (Table 1). As observed from the antifungal MIC values (0.2μg/mL), the compounds were fungicidal at significantly lower doses, 25 times lesser than cytotoxic IC50 values. Therefore, it may be inferred that the compounds do not inhibit the growth of normal cell lines (V79 and HBL100), at concentrations active for fungicidal action.
Apoptosis analysis by acridine orange and ethidium bromide staining (AO/EB staining) Method
Among the various compounds tested, the enhanced late apoptosis induced by the 5,7-dimethyl compound 3c and 7-methyoxy derivative 3f resembled the pattern exhibited by the standard doxorubicin. The lower percentage of cell death by Necrosis in all the compounds 3a-h, clearly indicated that they induced cell death mainly by Apoptosis after an exposure of 48 h. The compounds 3a-h along with standard drug (Doxorubicin) was able to significantly (p < 0.05) induce cell death in HeLa cell lines. It is evident that (Table 2) the compounds 3a-h induced cell death mainly by apoptosis.
Table 2: Quantitative comparison of the % apoptotic and necrotic cells induced doxorubicin (0.5μM) and test compounds (50μM) on HeLa cells.
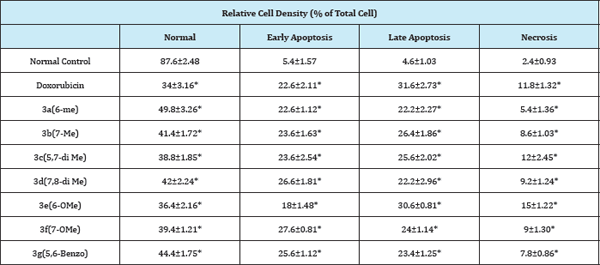
(*p<0.05 vs. Normal Control, n=6)The criteria for each component were determined as followings. (a) Normal cells: uniformly green stain; (b) Early apoptotic cells: stain green and contain bright green dots in the nuclei chromatin condensation & nuclear fragmentation; (c) Late apoptotic cells: incorporate ethidium bromide (orange), condensed and fragmented nucleus; (d) Necrotic cells: orange stain but with nuclear morphology resembling that of viable cells. Values were calculated from 8 different view fields in microscope (400*) using flourescein filter.
Anti- fungal screening
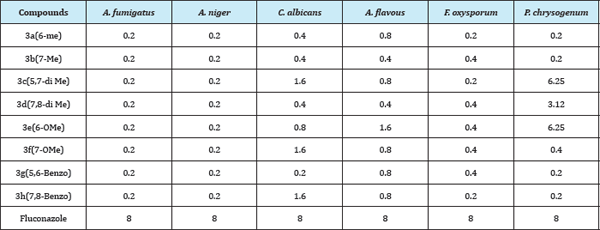
Anti-fungal screening of the synthesised compounds against six different strains viz., A. fumigatus, A. niger, C. albicans, A. flavous, F oxysporum and P. chrysogenum revealed that (Table 3) introduction of bis (2-chloroethyl) amino side chain at the allylic position of coumarin ring considerably increases the antifungal activity of the parent moiety irrespective of substituents on the coumarin ring. All the screened compounds showed excellent antifungal activity with IC50 of 0.2μg/mL against both A. fumigatus and A. niger. In case of C. albicans compound 3g showed highest activity with IC50 of 0.2μg/mL. Compounds 3b and 3d exhibited better activity with IC50 of 0.4μg/mL against A. flavous. For F. oxysporum compounds 3a, 3c and 3h were highly active with IC50 of 0.2μg/mL. Monomethyl substituted 3a and 3b and benzo substituted 3g and 3h compounds were highly active against P. chrysogenum with IC50 of 0.2μg/mL. In summary all the synthesised compounds were highly active against plant as well as human fungal pathogens and much better active compare to Fluconazole.
Table 3: Anti-fungal activity in μg/mL.
Anti-bacterial screening
All the synthesised title compounds were screened for both Gram-positive and Gram-negative bacteria. Coumarin derivatives are well known for anti-bacterial activities viz., Novobiocin. Even though Novobiocin is active against both Gram-positive and Gramnegative bacteria, it is quite specific for Gram-positive bacteria, in particular S. aureus (Wishnow et al. 1965). Antibacterial study of title compounds revealed that all the compounds are active against Gram-positive a bacterium, which is in agreement with the earlier reports, and are highly active against S. aureus. Compounds 3c and 3d showed very good activity with MIC of 3.12μg/mL. Compound 3b showed the best result with MIC of 0.2μg/mL against S. aureus (Table 4). Thus the introduction of bis(2-chloroethyl)amino side chain at allylic position of 2H-chromen-2-one has a significant effect on the antibacterial activity.
Anti-TB activity using Alamar Blue Dye
In view of the earlier reports on anti-tubercular activity of the coumarin derivatives, 4-((bis(2-chloroethyl)amino)methyl)- 2H-chromen-2-ones 3a-h were screened against M. tuberculosis to explore their activity. Compared to other coumarin derivatives [16], title compounds exhibited promising anti-tubercular activity with low MIC of 25μg/mL (Table 4) against M. tuberculosis. This enhancement in the activity is due to the introduction of the bis (2-chloroethyl)amino side chain at the allylic position of 2H-chromen-2-one ring.
Table 4: Anti-bacterial and anti-TB activity in μg/mL.
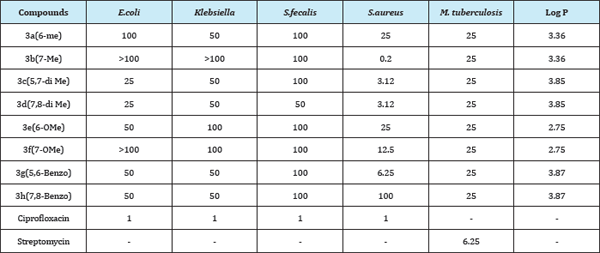
Computed Log P and Mechanism of Action
Log P is a measure of hydrophobicity which is important for the penetration, distribution and interaction of drug with receptors. It has an important role on the inhibitory activity which is usually related to pharmacological activity. In present study Log P plays an important role in determining the biological activity of coumarin nitrogen mustards. Within a homologous series, drug absorption usually increases as lipophilicity rises and is maintained at a plateau for a few units of Log P after which there may be a steady decrease, giving a parabolic relation. In general, Log P values between 0 and 3 constitute an optimal window for passive drug absorption. A Log P value below 0 means that the compound is hydrophilic, and hence it will have a good solubility but it may have poor permeability. Whereas, a Log P value far higher than 3 means that the compound is highly lipophilic, hence, tends to favour absorption, and renders the compounds more susceptible to metabolism. The influence of lipophilicity on the metabolic clearance of drugs is attributed mainly to the increased affinity of drugs for the enzymes [17].
In present study all the substituted coumarin nitrogen mustards possess Log P values in the range 2.5-4, which indicated that all the coumarin mustards are hydrophobic in nature. We had plotted the graph of Log P values of substituted coumarin nitrogen mustards (except benzo substituent) against corresponding IC values. Since C6 and C7 substituted coumarin nitrogen mustards showed activity with different trends we plotted the graphs separately. IC50 values of all the screened compounds showed parabolic graph with vertex at Log P= 3.36.
Anti-cancer activity
Both in case of HeLa and MCF-7, C6, and C7 substituted coumarin nitrogen mustards exhibited downward parabolic graph with vertex at Log P=3.36 (Figure 1).
Anti-fungal activity
All the substituted coumarin nitrogen mustards showed excellecnt anti-fungal activity with same extent against A. fumigatus and A. niger. For rest of the fungal strains screened compounds showed both upward and downward parabolic graph with vertex at Log P=3.36 Figure 1.
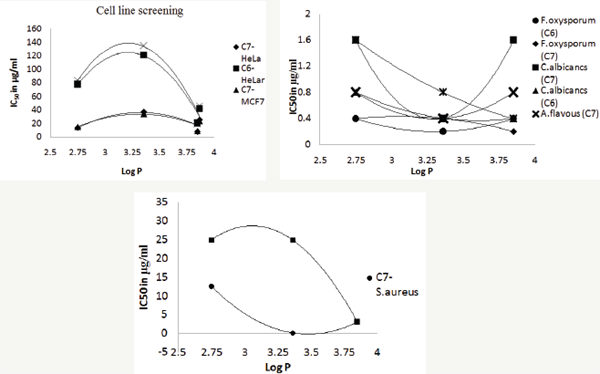
Figure 1: a. Variation of Log P with the cell-line activity of coumarin nitrogen mustards.
b. Variation of Log P with the anti-fungal activity of coumarin nitrogen mustards.
c. Variation of Log P with the anti-bacterial activity of coumarin nitrogen mustards.
Anti-bacterial
Screened coumarin nitrogen mustards were active against S. aurues, C6 and C7 substituted coumarin nitrogen mustards showed downward and upward parabolic graphs respectively with vertex at Log P=3.36 Figure 1.
Electronic effect with reference to anti-TB, anti-fungal, anti-cancer and other anti-microbial activity
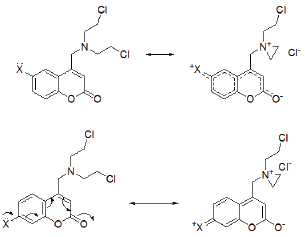
Importance of n electron delocalization leading to charge separated structures has been emphasized by Kumler [18] in rationalizing the anti microbial activity of sulphonamides. It has been applied to rationalise the activity of warfarin, phenyl butazone and metformin as well [19]. In recent years the antimicrobial activity of quaternary nitrogen compounds possessing long chain alkyl groups with surfactant properties has also been reported [20]. The presence of polar covalent bonds leads to greater contribution for charge separated structure even by a-electron delocalization due to inductive withdrawal of electrons. Such structures have greater ability to cross the membrane barrier in biological systems [21]. The presently synthesized compounds possessing groups at C6 and C7 positions can effectively lead to significant contribution by charge separated structures (Figure 2). It is also pertinent to mention that neighboring group participation by nitrogen lone pair can lead to quaternary aziridinium ions. Thus a unique contribution of n electron delocalization and in situ generated quaternary ammonium salts probably contribute to greater antimicrobial activity of the presently synthesized compounds (Figure 2).
Figure 2: Role of electronic effect in C6 and C7-substituted coumarins.
Structure Activity Relation
Figure 3: Electron delocalisation in C5 and C7-substituted coumarins.
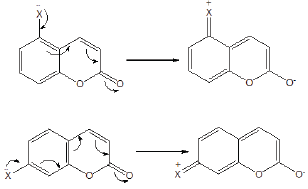
Electron donating substituents at C7 and C5 positions were found to be more effective than at C6 position. Amongst the methyl and methoxy groups, methoxy substituted compound was more effective than the methyl substituted compound. Presence of electron donating group at C7 and C5 position enhances the extent of electron delocalisation by extending the resonance through the pyran ring which further results in the formation of charge separated structure which actually influences the biological activity of the compounds (Figure 3).
Anti-cancer activity: C7-methoxy substituted compound 3f was found to be more effective than the C7-methyl substituted derivative 3b, where in former exhibited lower IC50 values of 13.83μM/mL and 15.50μM/mL and later exhibited 37.15μM/mL and 34.41μM/ mL against HeLa and MCF-7 cell lines respectively. 5,7-dimethyl substituted compound 3c was found to be the most potent in the series exhibited lowest IC50 values of 7.74μM/mL and 9.11μM/mL against HeLa and MCF-7 cell lines respectively.
Anti-fungal activity
All the synthesised coumarin nitrogen mustards showed excellent antifungal activity against A. fumigatus and A. niger irrespective of substituents on coumarin ring. In case of A.flavous and P. chrysogenum C7-methyl substituted compound 3b showed better activity compare to C6-methyl substituted compound 3a. Same trend was followed by methoxy substituted compounds, compound 3f showed good activity than compound 3e.
Anti-bacterial activity
Coumarin nitrogen mustards were active against S. aureus where in C7-methyl and methoxy substituted compounds 3b and 3f showed remarkably higher activity compare to C6-methyl and methoxy substituted compounds 3a and 3e.
Anti-tubercular activity
All the compounds showed moderate activity against M. tuberculosis irrespective of substituents on the coumarin moiety.
Experimental Protocols
General materials and methods
The melting points were determined by open capillary method and are uncorrected. The IR spectra (KBr disc) were recorded on a Nicolet-5700 FT-IR spectrophotometer. JH-NMR spectra were recorded on Bruker 300 MHz spectrometer using CDCl3 and DMSO-d, as solvents and TMS as an internal standard. The chemical 6 shifts are expressed in δ ppm. The mass spectra were recorded using Agilent-single Quartz GC-MS. The elemental analysis was carried out using Heraus CHN rapid analyzer. The purity of the compound was checked by T.L.C. All the chemicals purchased were of analytical grade, and were used without further purification unless otherwise stated. Log P values were calculated using Chem Draw Ultra 8.0.
Synthesis
General procedure for the synthesis of substituted 4-(bromomethyl)-2H-chromen-2-ones (1a-h): The required substituted 4-(bromomethyl)-2H-chromen-2-ones [22] have been synthesized by the Pechmann cyclization of substituted phenols (A) with ethyl 4-bromo-3-oxobutanoate (B) [23].
General procedure for the synthesis of 4-{[bis (2-hydroxyethyl) amino] methyl} -2H-chromen-2-ones (2a-h): Substituted 4-(bromomethyl)-2H-chromen-2-ones (1a-h) (0.01 mol) and diethanol amine (0.015mol) was taken in a 1:1 (25mL) mixture of ethyl alcohol and ethyl acetate. Reaction mixture was refluxed for 5-6 h and after completion of the reaction (monitored by TLC) solvent was evaporated under vacuum. The obtained viscous mass was diluted with ice-cold water, extracted with ethyl acetate and washed with water. The organic layer was dried over anhydrous magnesium sulphate and concentrated. The product was purified by column chromatography using hexane/ethyl acetate (6:4) as eluting solvent.
4-{[bis (2-hydroxyethyl) amino] methyl}-6-methyl-2H- chromen-2-one (2a): Colorless solid; yield 80%; m.p. 104-6 °C; IR (KBr) cm 1 1710 (C=O), 3387 (OH); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.00 (s, br, 1H, OH, D2O exchangeable), 2.4-2 (s, 3H, C6-CH3), 2.82 (t, 4H, J = 6Hz, 2N-CH2), 3.22 (s, br, 1H, OH, D2O exchangeable;), 3.70 (t, 4H, J = 6Hz, 2CH2-OH), 3.92 (s, 2H, C4-CH2), 6.71 (s, 1H, C;-H), 7.21 (d, 1H, J = 9Hz, Ar-C8-H), 7.32 (d, 1H, J = 9Hz, Ar-C7-H), 7.56 (s, 1H, Ar-C5-H). MS m/z (M+1) = 278. Anal. Calcd for C15H19NO4 (%): Calcd. C, 64.97; H, 6.91; N, 5.05. Found: C, 64.84; H, 6.78; N, 4.79. % of eluting solvent = hexane/ethyl acetate (6:4).
4-{[bis (2-hydroxyethyl) amino] methyl}-7-methyl-2H- chromen-2-one (2b): Colorless solid; yield 82%; m.p. 100-2 °C; IR (KBr) cm-1 1711 (C=O), 3382 (OH); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.00 (s, br, 1H, OH, D2O exchangeable), 2.42 (s, 3H, C6-CH3), 2.82 (t, 4H, J = 6Hz, 2N-CH2), 3.22 (s, br, 1H, OH, D2O exchangeable;), 3.70 (t, 4H, J = 6Hz, 2CH2-OH), 3.92 (s, 2H, C4-CH2), 6.71 (s, 1H, C;- H), 7.20 (Ar-C8-H), 7.42 (d, 1H, J = 9Hz, Ar-C6-H), 7.56 (d, 1H, J = 9Hz, Ar-C5-H). MS m/z (M+1) = 278. Anal. Calcd for C15H19NO4 (%): Calcd. C, 64.97; H, 6.91; N, 5.05. Found: C, 64.84; H, 6.78; N, 4.79. % of eluting solvent = hexane/ethyl acetate (6:4).
4-{[bis (2-hydroxyethyl) amino] methyl}-5, 7-dimethyl-2H- chromen-2-one (2c): Colorless solid; yield 68%; m.p. 102-4 °C; IR (KBr) cm-1 1711 (C=O), 3444 (OH); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.34 (s, 3H, CH3), 2.59 (s, 1H, -OH), 2.67 (s, 3H, CH3), 2.83(d, 4H, J = 6Hz, 2N-CH2), 3.69 (t, 4H, J = 6Hz, 2CH2-OH), 4.04 (s, 2H, C4- CH2), 5.05 (s, br, 1H, OH), 6.88 (s, 1H, C3-H), 6.89 (s, 1H, Ar-H), 6.96 (s, 1H, Ar-H). MS m/z (M+1) = 292. Anal. Calcd for C16H21NO4 (%): Calcd. C, 65.96; H, 7.27; N, 4.81. Found: C, 65.84; H, 7.18; N, 4.79. % of eluting solvent = hexane/ethyl acetate (6:4). 4-{[bis (2-hydroxyethyl) amino] methyl}-7, 8-dimethyl- 2H-chromen-2-one (2d): Yellow solid; yield 73%; m.p. 92-94 °C; IR (KBr) cm-1 1715 (C=O), 3404 (OH); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.35 (s, 3H, CH;), 2.37 (s, 3H, CH;), 2.81 (t, 4H, J = 6Hz, 2N-CH2), 3.04 (s, br, 1H, ,OH, D2O exchangeable), 3.68 (t, 4H, J = 6Hz, 2CH2-OH), 3.89 (s, 2H, C4-CH2), 5.33 (s, br, 1H, OH, D2O exchangeable), 6.63 (s, 1H, C3-H), 7.09 (d, J = 6Hz, 1H,C6-H), 7.52 (d, 1H, J = 9Hz, C5-H). MS m/z (M) = 291. Anal. Calcd for C16H21NO4 (%): Calcd. C, 65.96; H, 7.27; N, 4.81. Found: C, 65.74; H, 7.08; N, 4.68. % of eluting solvent = hexane/ethyl acetate (6:4).
4-{[bis (2-hydroxyethyl) amino] methyl}-6-methoxy -2H-chromen-2-one (2e): Yellow solid; yield 70%; m.p. 98-100 °C; IR (KBr) cm-1 1716 (C=O), 3319 (OH); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.15 (s, br, 1H, OH, D2O exchangeable), 2.532 (t, 4H, J = 6Hz, 2N-CH2), 3.34 (s, br, 1H, OH, D2O exchangeable ), 3.69 (t, 4H, J = 6Hz, 2CH2-OH), 3.86 (s, 3H, C6-OCH3), 3.89 (s, 2H, C4-CH2), 6.67 (s, 1H, C3-H), 7.09 (d, 1H, J = 9Hz, Ar-H), 7.23-7.28 (m, 2H, Ar-H). MS m/z (M+1) = 294. Anal. Calcd for C15H19NO5 (%): Calcd. C, 61.42; H, 6.53; N, 4.78. Found: C, 61.28; H, 6.39; N, 4.62. % of eluting solvent = hexane/ethyl acetate (7:3).
4-{[bis (2-hydroxyethyl) amino] methyl}-7-methoxy -2H-chromen-2-one (2f): Yellow solid; yield 73%; m.p. 118-120 °C; IR (KBr) cm-1 1705 (C=O), 3408 (OH); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.40 (s, 2H, -OH, D2O exchangeable), 2.81 (t, 4H, J = 6Hz, 2N-CH2), 3.69 (t, 4H, J = 6Hz, 2CH2-OH), 3.86-3.88 (m, 5H, C4-CH2, C7- OCH3), 6.51 (s, 1H, C3-H), 6.78 (d, 1H, J = 2.2Hz, Ar-H), 6.83(dd, 1H, J = 2.4Hz, 8.8Hz, Ar-H), 7.70 (d, 1H, J = 8.8Hz, Ar-H). MS m/z (M+1) = 294. Anal. Calcd for C15H19NO5 (%): Calcd. C, 61.42; H, 6.53; N, 4.78. Found: C, 61.38; H, 6.23; N, 4.54. % of eluting solvent% of eluting solvent = hexane/ethyl acetate (7:3).
1-{[bis (2-hydroxyethyl) amino] methyl}-3H-benzo[f] chromen-3-one (2g): Yellow solid; yield 72%; m.p. 140-42 °C; IR (KBr) cm-1 1711 (C=O), 3388 (OH); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 1.87 (s, br, 2H, OH, D2O exchangeable), 2.90 (t, 4H, J = 6Hz, 2N-CH2), 3.71 (t, 4H, J = 6Hz, 2CH2-OH), 4.36 (s, 2H, C4-CH2), 7.05 (s, 1H, C3-H), 7.26 (s, 1H, Ar-H), 7.46-7.99 (m, 4H, Ar-H), 8.53 (d, 1H, J = 9Hz, Ar-H). MS m/z (M+1) = 314. Anal. Calcd for C18H19NO4 (%): Calcd. C, 68.99; H, 6.11; N, 4.47. Found: C, 68.74; H, 6.05; N, 4.28. % of eluting solvent = hexane/ethyl acetate (6:4).
4-{[bis (2-hydroxyethyl) amino] methyl}-2H-benzo[h] chromen-2-one (2h): Yellow solid; yield 74%; m.p. 174-76 °C; IR (KBr) cm-1 1714 (C=O), 3369 (OH); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 1.66 (s, br, 1H, OH, D2O exchangeable), 2.68 (s, br, 1H, OH, D2O exchangeable), 2.87 (t, 4H, J=6Hz, 2N-CH2), 3.70 (t, 4H, J=6Hz, 2CH2-OH), 4.02 (s, 2H, C4-CH2), 6.73 (s, 1H, C3-H), 7.64-8.56 (m, 6H, Ar-H). MS m/z (M+1)=314. Anal. Calcd for C18H19NO4 (%): Calcd. C, 68.99; H, 6.11; N, 4.47. Found: C, 68.91; H, 6.07; N, 4.39. % of eluting solvent=hexane/ethyl acetate (6:4).
General procedure for the synthesis
4-{[bis (2-chloroethyl) amino] methyl}-2H-chromen-2- ones (3a-h): Substituted 4-{[bis (2-hydroxyethyl) amino] methyl}- 2H-chromen-2-ones (2a-h) (0.01mol) was taken in a two necked R. B. flask with dropping funnel and reflux condenser containing a calcium chloride guard tube and the reaction mixture was cooled to 0 °C. Thionyl chloride (0.03mol) was added through a dropping funnel in portion wise with occasional shaking so that temperature should not rise above 0 °C. The reaction mixture was heated to 60-70 °C for 15minutes (monitored by TLC), cooled and poured on to crushed ice (50g). The separated solid was filtered off, washed with water and dried. Compound was purified by column chromatography using hexane/ethyl acetate (9:1) as eluting solvent.
4-{[bis (2-chloroethyl) amino] methyl}-6-methyl-2H- chromen-2-one (3a): Colorless solid; yield 92%; m.p. 164-6 °C; IR (KBr) cm-1 1703 (C=O); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.34 (s, 3H, C6-CH3), 2.97 (t, 4H, J = 6Hz,32N-CH2), 3.50 (t, 4H, J = 6Hz, 2CH2-Cl), 3.89 (s, 2H, C4-CH2), 6.56 (s, 1H, C3-H), 7.16-7.28 (m, 2H, Ar-H), 7.52 (s, 1H, Ar-H). 13C NMR (CDCl3, 300 MHz, TMS): δ ppm 21.35, 42.09, 56.36, 57.00, 114.97, 1153.08, 117.25, 118.44, 124.59, 134.30, 152.19, 152.76, 161.56. MS m/z (M, M+2, M+4) = 313(9:6:1). Anal. Calcd for C15H17Cl2NO2 (%): Calcd. C, 57.34; H, 6.91; N, 4.46. Found: C, 57.23; H, 6.83; N, -4.37. % of eluting solvent = hexane/ethyl acetate (6:4)
4-{[bis (2-chloroethyl) amino] methyl}-7-methyl-2H- chromen-2-one (3b): Colorless solid; yield 87%; m.p. 90-92 °C; IR (KBr) cm-1 1722 (C=O); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.38 (s, 3H, C7-CH3), 2.97 (t, 4H, J = 6Hz, 2N-CH2), 3.49 (t, 4H, J = 6Hz, 2CH2-Cl), 3.88 (s, 2H, C4-CH2), 6.51 (s, 1H, C3-H), 7.02 (d, 1H, J = 9 Hz, Ar-H), 7.09 (s, 1H, Ar-H), 7.58 (d,1H, J = 9Hz, Ar-H). 13C NMR (CDCl3, 300 MHz, TMS): δ ppm 22.03, 42.08, 56.38, 56.99, 114.03, 116.32, 117.72, 124.40, 125.79, 143.54, 152.89, 154.20, 161.59. MS m/z (M, M+2, M+4) = 313 (9:6:1). Anal. Calcd for C15H17Cl2NO2 (%): Calcd. C, 57.34; H, 6.91; N, 4.46. Found: C, 57.30; H, 6.88; N, 4.37.
4-{[bis (2-chloroethyl) amino] methyl}-5,7-dimethyl-2H- chromen-2-one (3c): Colorless solid; yield 94%; m.p. 104-6 °C; IR (KBr) cm-1 1706 (C=O); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.30 (s, 3H, C7-CH3), 2.61 (s, 3H, C5-CH3), 33.00 (t, 4H, J = 6Hz, 2N-CH2), 3.51 (t, 4H, J = 6Hz, 2CH2-Cl), 4.07 (s, 2H, C4-CH2), 6.76 (s, 1H, C3-H), 6.83 (s, 1H, C6-H), 6.95 (s, 1H, C8-H). MS m/z (M, M+2, M+4) = 327 (9:6:1). Anal. Calcd for C16H19Cl2NO2 (%): Calcd. C, 58.55; H, 5.83; N, 4.27. Found: C, 58.43; H, 5.79; N, 4.19.
4-{[bis (2-chloroethyl) amino] methyl}-7, 8--dimethyl-2H- chromen-2-one (3d): Colourless solid; yield 94%; m.p. 68-70 °C; IR (KBr) cm-1 1716 (C=O); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.38 (s, 3H, CH3), 2.39 (s, 3H, CH3), 3.04 (t, 4H, J = 6Hz, 2N-CH2), 3.56 (t, 4H, J = 6Hz, 2CH2-Cl), 3.95 (s, 2H, C4-CH2), 6.60 (s, 1H, C3-H), 7.09 (d, 1H, J = 6Hz, C6-H), 7.50 (d, 1H, J = 6Hz, C5-H). 13C NMR (CDCl3, 300 MHz, TMS): δ ppm 12.07, 20.75, 31.33, 42.08, 56.43, 57.05, 113.62, 116.46, 121.32, 125.29, 125.99, 142.06, 152.32, 153.25, 161.80. MS m/z (M, M+2, M+4) = 327 (9:6:1). Anal. Calcd for C16H19Cl2NO2 (%): Calcd. C, 58.55; H, 5.83; N, 4.27. Found: C, 58.43; H, 5.77; N, 4.18.
4-{[bis (2-chloroethyl) amino] methyl}-6-methoxy-2H- chromen-2-one (3e): Colourless solid; yield 88%; m.p. 148-150 °C; IR (KBr) cm-1 1709 (C=O); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 2.97 (t, 4H, J = 6Hz, 2N-CH2), 3.51 (t, 4H, J = 6Hz, 2CH2-Cl), 3.80 (s, 3H, C6-OCH3), 3.88 (s, 2H, C4-CH2), 6.55 (s, 1H, C3-H), 7.05 (dd, 1H, J = 3Hz, 9Hz, Ar-H), 7.21(dd, 2H, J = 3Hz, 9Hz, Ar-H). 13C NMR (CDCl3, 300 MHz, TMS): δ ppm 42.14, 56.38, 56.85, 56.93, 107.66, 107.738, 115.74, 115.83, 118.45, 119.18, 119.81, 148.48, 153.42, 156.34, 161.48. MS m/z (M, M+2, M+4) = 329 (9:6:1). Anal. Calcd for C15H17Cl2NO3 (%): Calcd. C, 54.56; H, 5.19; N, 4.24. Found: C, 54.43; H, 5.13; N, 4.16.
4-{[bis (2-chloroethyl) amino] methyl}-7-methoxy-2H- chromen-2-one (3f): Colourless solid; yield 89%; m.p. 80-82 °C; IR (KBr) cm-1 1708 (C=O); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 3.2 (t, J = 8Hz, 4H, 2N-CH2), 3.56 (t, 4H, J = 8Hz, 2CH2-Cl), 3.87(s, 3H, C7-OCH3), 3.92 (s, 2H, C4-CH2), 6.47 (d, 1H, J = 1.2 Hz, C3-H), 6.85 (d, 2H, J = 8.4 Hz, Ar-H), 7.72 (d, 1H, J = 8.4 Hz, Ar-H). 13C NMR (CDCl3, 300 MHz, TMS): δ ppm 41.98, 56.39, 56.84, 101.24, 101.31, 111.638, 111.89, 112.22, 112.57, 125.88, 125.94, 153.16, 155.83, 161.68, 163.02. MS m/z (M, M+2, M+4) = 329 (9:6:1). Anal. Calcd for C15H17Cl2NO3 (%): Calcd. C, 54.56; H, 5.19; N, 4.24. Found: C, 54.37; H, 5.14; N, 4.18.
4-{[bis (2-chloroethyl) amino] methyl}-3H-benzo[f] chromen-3-one (3g): Yellow solid; yield 86%; m.p. 74-6°C; IR (KBr) cm-1 1727 (C=O); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 3.05 (t, 4H, J = 6Hz, 2N-CH2), 3.55 (t, 4H, J = 6Hz, 2CH2-Cl), 4.35 (s, 2H, C4-CH2), 7.03 (s, 1H, C3-H), 7.39-8.33 (m, 6H, Ar-H). MS m/z (M, M+2, M+4) = 349 (9:6:1). Anal. Calcd for C18H17Cl2NO2 (%): Calcd. C, 61.73; H, 4.89; N, 4.00. Found: C, 61.67; H, 4.82; N, 3.96.
4-{[bis (2-chloroethyl) amino] methyl}-2H-benzo[h] chromen-2-one (3h): Yellow solid; yield 89%; m.p. 116-18 °C; IR (KBr) cm-1 1713 (C=O); 1H NMR (CDCl3, 300 MHz, TMS): δ ppm 3.2 (t, 4H, J = 6Hz, 2N-CH2), 3.52 (t, 4H, J = 6Hz, 2CH2-Cl), 4.00 (s, 2H, C4-CH2), 6.67 (s, 1H, C3-H), 7.57-8.54 (m, 6H, Ar-H). MS m/z (M, M+2, M+4) = 349 (9:6:1).. Anal. Calcd for C18H17Cl2NO2 (%): Calcd. C, 61.73; H, 4.89; N, 4.00. Found: C, 61.69; H, 4.83; N, 3.92.
Pharmacology
Cell line screening MCF-7 and HeLa: MCF-7 (human breast adenocarcinoma) and HeLa (human cervical tumor cells) cells procured from the National Center for Cell Science, Pune, India were sub-cultured every two to three days and maintained in 25 cm2 tissue culture flasks (Tarsons Products, Kolkata, India) containing MEM medium supplemented with 10% fetal bovine serum (FBS) and 50μg/mL gentamicin sulfate at 37 °C in a CO2 incubator (NuAire, Plymouth,USA) in an atmosphere of humidified 5% CO2 in 95% air(26).
Cytotoxic Study
The cytotoxic effect of drugs on normal cells (V79 and HBL100) was assessed by MTT assay. In brief, exponentially growing cells (1 x 104 cells/well) were plated in 96-well plates and allowed to adhere for 24 h prior to extract addition. The drugs were dissolved in 0.1% DMSO and then diluted with the medium. The cells were then exposed to different concentrations of drug (5-200μg/mL) for 24h. The cells in the control wells received medium containing the same volume of DMSO (0.1%). After the incubation, 100μL of MTT reagent (1mg/mL in PBS) was added and cells were incubated for an additional 4 h. The formazan produced by the viable cells was solubilized by addition of 100μL DMSO. The suspension was placed on a micro-vibrator for 5 min and absorbance was recorded at 540 nm by the plate reader (ELx800, BioTek, VT, USA). The experiment was performed in triplicate. Doxorubicin was used as positive control [24]. The percentage of growth inhibition was calculated with respect to vehicle control using the formula
% Inhibition = [{(Control absorbance - Blank absorbance) - (Test absorbance - Blank absorbance)} / (Control absorbance - Blank absorbance)] x 100
Apoptosis analysis by acridine orange and ethidium bromide staining (AO/EB staining) Method
Acridine orange/ethidium bromide (AO/EB) staining of HeLa cells was done to observe the apoptotic pattern induced by the test compounds 3a-h according to previously described procedure with suitable modifications. In brief, cells were seeded in 6-well plates for 24h and then treated with test compounds (50μM) for 48 h. After harvesting by trypsinisation, cells were washed with 1xPBS. The cell suspension (25μL of 106 number cells/mL) with 1μL AO/ EB solution (1 part of 100μg/mL AO in PBS; 1 part of 100μg/mL EB in PBS), each sample was mixed just prior to the microscopic examination and quantification. The cell suspension (10μL) was placed on a microscopic slide, covered with a glass cover slip, and at least 300 cells were examined under a fluorescence microscope using a flourescein filter [25].
Anti-microbial screening
Nine dilutions of each drug have to be done with BHI for MIC. In the initial tube 20μL of drug was added into the 380μL of BHI broth. For dilutions 200μL of BHI broth was added into the next 9 tubes separately. Then from the initial tube 200μL was transferred to the first tube containing 200μL of BHI broth. This was considered as 10-1 dilution. From 10-1 diluted tube 200μL was transferred to second tube to make 10-2 dilution. The serial dilution was repeated up to 10-9 dilution for each drug. From the maintained stock cultures of required organisms, 5μL was taken and added into 2mL of BHI (brain heart infusion) broth. In each serially diluted tube 200μL of above culture suspension was added. The tubes were incubated for 24 h and observed for turbidity [26].
Anti-TB activity using Alamar Blue Dye
Anti mycobacterial activity of compounds were assessed against M. tuberculosis using microplate Alamar Blue assay (MABA). This methodology is non-toxic, uses a thermally stable reagent and shows good correlation with propotional and BACTEC radiometric method. Briefly, 200μL of sterile deionzed water was added to all outer perimeter wells of sterile 96 wells plate to minimized evaporation of medium in the test wells during incubation. The 96 wells plate received 100μL of the Middlebrook 7H9 broth and serial dilution of compounds were made directly on plate. The final drug concentrations tested were 100-0.2μg/mL. Plates were covered and sealed with parafilm and incubated at 37 °C for five days. After this time, 25μL of freshly prepared 1:1 mixture of Almar Blue reagent and 10% between 80 was added to the plate and incubated for 24h. A blue colour in the well was interpreted as no bacterial growth, and pink colour was scored as growth. The MIC was defined as lowest drug concentration which prevented the colour change from blue to pink [27].
Conclusion
In conclusion all the title compounds have shown moderate activity against MCF-7 and HeLa cell lines and induced cell death mainly by apoptosis. Their specificity towards gram positive species is quite significant and the most active compound in this series is 3b. The results obtained with six fungal strains are quite encouraging. Title compounds showed promising anti-tubercular and antibacterial activity. Though the two armed side chain is no longer a group of choice for anti-cancer activity, we have discovered its potential as a fungicidal pharmacophore which warrants further investigation.
Acknowledgement
Reshma Naik is grateful to DST, New Delhi, India for the financial assistance in the form of INSPIRE Fellowship. We thank University Scientific Instrumentation Center (USIC), Karnatak University Dharwad for IR, NMR, MS and analytical data.
References
- Van Duuren BL, Goldschmidt BM (1966) Carcinogenicity of epoxides, lactones, and peroxy compounds. 3. Biological activity and chemical reactivity. J Med Chem 9(1): 77-79.
- Shi Y, Zhou CH (2011) Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg Med Chem Lett 21(3): 956-960.
- Montagner C, Souza SM, Groposoa C, Delle Monache F, Smania EF, et al.(2008) Anti-fungal activity of coumarins. Journal of biosciences 63(1-2): 21-28.
- Hwu JR, Singha R, Hong SC, Chang YH, Das AR, et al. (2008) Synthesis of new benzimidazole-coumarin conjugates as anti-hepatitis C virus agents. Antiviral Res 77(2): 157-162.
- Cardoso SH, Barreto MB, Lourenco MC, Henriques M, Candea AL, et al. (2011) Anti-tubercular activity of new coumarins. Chemical biology & drug design 77(6): 489-493.
- Huang F, Tang LH, Yu LQ, Ni YC, Wang QM, et al. (2006) In vitro potentiation of antimalarial activities by daphnetin derivatives against Plasmodium falciparum. Biomed Environ Sci 19(5): 367-370.
- Kontogiorgis CA, Hadjipavlou-Litina DJ (2004) Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg Med Chem Lett 14(3): 611-614.
- Jung JC, Lee JH, Oh S, Lee JG, Park OS (2004) Synthesis and antitumor activity of 4-hydroxycoumarin derivatives. Bioorg Med Chem Lett 14(22): 5527-5531.
- Kostova I, Raleva S, Genova P, Argirova R (2006) Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg Chem Appl 2006:68274.
- Lacy A, Kennedy OR (2004) Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des 10(30): 3797-3811.
- Kulkarni MV, Kulkarni GM, Lin CH, Sun CM (2006) Recent advances in coumarins and 1-azacoumarins as versatile biodynamic agents. Curr Med Chem 13(23): 2795-2818.
- Bhattacharyya SS, Paul S, Mandal SK, Banerjee A, Boujedaini N, et al.(2009) A synthetic coumarin (4-Methyl-7 hydroxy coumarin) has anticancer potentials against DMBA-induced skin cancer in mice. Eur J Pharmacol 614(1-3): 128-136.
- Weber US, Steffen B, Siegers CP (1998) Antitumor-activities of coumarin,7- hydroxy-coumarin and its glucuronide in several human tumor cell lines. Res Commun Mol Pathol Pharmacol 99(2): 193-206.
- Khan IA, Kulkarni MV, Sun CM (2005) One pot synthesis of oxygenated tri-heterocycles as anti-microbial agents. Eur J Med Chem 40(11): 11681172.
- Laurin P, Ferroud D, Schio L, Klich M, Dupuis-Hamelin C, et al. (1999) Structure-activity relationship in two series of aminoalkyl substituted coumarin inhibitors of gyrase B. Bioorg Med Chem Lett 9(19): 28752880.
- Arshad A, Osman H, Bagley MC, Lam CK, Mohamad S, et al. (2011) Synthesis and antimicrobial properties of some new thiazolyl coumarin derivatives. Eur J Med Chem 46(9): 3788-3794.
- Panchagnula R, Thomas NS (2000) Biopharmaceutics and pharmacokinetics in drug research. Int J Pharm 201(2): 131-150.
- Daniels TC (1943) Synthetic Drugs. Annual Review of Biochemistry 12(1): 447-472.
- Logie L, Harthill J, Patel K, Bacon S, Hamilton DL, et al. (2012) Cellular responses to the metal-binding properties of metformin. Diabetes 61(6): 1423-1433.
- Ahmed SM, Ismail DA (2008) Synthesis and Biological Activity of 8- Hydroxyquinoline and 2-Hydroxypyridine Quaternary Ammonium Salts. Journal of Surfactants and Detergents 11(3): 231-235.
- Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, et al. (2002) Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J Med Chem 45(12): 2615-2623.
- Kulkarni MV, Patil VD (1981) Studies on coumarins, I. Arch Pharm (Weinheim) 314(8): 708-711.
- Burger A, Ullyot GE (1947) Analgesic studies. ß-ethyl and ß-isopropylamine derivatives of pyridine and thiazole1. J Org Chem 12(2): 342-355.
- Manjula SN, Kenganora M, Parihar VK, Kumar S, Nayak PG, et al. (2010) Antitumor and antioxidant activity of polyalthia longifolia stem bark ethanol extract. Pharm Biol 48(6): 690-696.
- Ribble D, Goldstein NB, Norris DA, Shellman YG (2005) A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol 5: 12.
- Schwalbe LR, Steele-Moore, AC Goodwin (2007) Anti-microbial susceptibility testing protocols. Crc Press, Florida, USA. p. 432.
- Lourenfo MCS, Marcus VN de S, Alessandra CP, Marcelle de LF, Raoni SBG, et al. (2007) Evaluation of anti-tubercular activity of nicotinic and isoniazid analogues. ARKIVOC (xv) 181-191.
© 2017 Naik RJ, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






