- Submissions

Full Text
Intervention in Obesity & Diabetes
Potential Antihyperglycemic, Antihypolipidemic and Antioxidant Effects of Aqueous Extract of Boscia Senegalensis (Capparidaceae) on Diet-Induced Hyperlipidemia in Rat
Faustin Dongmo1*, Elie Baudelaire Djantou2, Alcherif Hamid Mahamat3, Didier Beyssiri1, Selestin Sokeng Dongmo1 and Nicolas Njintang Yanou1,2
1Faculty of Science, Department of Biological Sciences, University of Ngaoundere, Cameroon
2Department of Food Science and Nutrition, ENSAI, University of Ngaoundere, Cameroon
3Department of Biomedical and Pharmacetical Sciences of National Higher Institute of Science and Techniques of Abeche, Chad
*Corresponding author:Faustin Dongmo, Department of Biological Sciences, Faculty of Science, University of Ngaoundere, PO Box 454, Ngaoundere, Cameroon
Submission:October 26, 2023;Published: December 05, 2023

ISSN 2578-0263Volume6 Issue3
Abstract
The effects of Boscia senegalensis seed decoction a potential medicinal tree used traditionally on the treatment of diabetes was evaluated on serum lipid profile, glycemic and on the antioxidant, property was evaluated. This study was carried out at the laboratory of medicinal plants, health and galelic formulation of the Department of biological sciences, Faculty of Science, University of Ngaoundere, during the period started from the 1st April 2022 to the 1st June 2022. To achieve our goals, our study was performed on male rats feeding simultaneously with Hyperlipidemic Diet (HD) and the extract of Boscia senegalensis for a period of 8 weeks. The measuring of fasting blood glucose and the measure of the lipid profile has been taken as the major marker of anti hyperlipidemia. At the beginning of the experiment the mean fasting blood glucose levels (83.7±1.9mg/mL) were not significantly different. In rat fed normal standard diet, the blood glucose was constant to 84.4±1.9mg/mL during the 7 weeks. It was found that Boscia senegalensis extract prevented simultaneously weight gain, increased blood sugar and increased blood lipids induced by consumption of a high fat diet the extract facilitates the elimination of lipids in the stool. In normal control group the levels of SOD, CAT and MDA were 6.6-9.7μU/mg, 39.2-44.0U/mg et1.1-13μM in plasma, liver and kidney respectively. Irrespective of the diet and drug administration regimes, stress parameters were lower in the blood and higher in the kidney. The extract decreasing blood levels of alanine aminotransferase (ALAT/ASAT) and serum creatinine, respectively. Boscia. senegalensis extract has an antihyperlipidemic properties and can be used in the treatment of vascular disorders including atherosclerosis.
Keywords:Boscia senegalensis; Antihyperlipidemic; Antihyperglycemic; Hyperlipidemic diet; Antioxidant
Introduction
Obesity is the central cause of metabolic syndrome [1]. Also called multifactor risk syndrome, obesity is generally characterized by hyperglycemia, hyperlipidemia, oxidative stress and hypertension whit is a significant factor risk of cardiovascular diseases [2]. Nowadays there is a growing increase in obesity diabetes and cardiovascular diseases in the world and these diseases are responsible for more than 30% deaths in the world [3]. One of the more important causes of obesity and type II diabetes is lifestyle characterized by a high energy diet. This has allowed scientists to develop a model of metabolic syndrome rats obtained by submitting to the rat’s high diet content of saturated fatty acids and cholesterol. It has been demonstrated that high fat diets decrease lecithin-cholesterol-acyl-transferase activity, therefore prevent reverse transport of cholesterol to the liver for catabolism and the high fat diets also reduces the LDL receptor activity and increase the hepatic synthesis of LDL cholesterol [4,5]. Likewise, the high fat-cholesterol diet also induced in 6 weeks dyslipidemia characterized by the accumulation of fat in the kidney, heart and liver [6].
Dyslipidemia is a primary or secondary pathological change in serum lipids. It is a metabolic and chronic abnormality characterized by a persistent elevation of triglyceride, LDL-cholesterol and a decrease in HDL cholesterol [7]. This anomaly contributes to the development of atherosclerosis. Their causes can be primitive and therefore genetic, or secondary to pathology. The diagnosis of dyslipidemia is based on the determination of plasma levels of cholesterol and triglycerides [8]. Hyperglycemia is a condition characterized by elevated blood glucose levels following a decreased biological response of peripheral tissues to insulin secretion. Hyperglycemia may be pathological such as in the case of dyslipidemia [9,10]. In addition, hyperglycemia may be promoted by exposure of cells to oxidative stress [11]. In the latter, we have to modify the transcription of glucose transporters, and the level of GLUT-1 is increased while GLUT-4 is reduced [12]. The activation of cytoplasmic protein kinase causes the phosphorylation of serine residues and the inactivation of hormone receptors, which inhibits the transmission of the insulin signal and the cellular uptake of glucose [13,14].
The management of dyslipidemia is the key point in the treatment of obesity, and statins represent the first line of drug treatment [9]. One of these is atorvastatin, an inhibitor of 3-Hydroxy- 3-Methylglutaryl CoA reductase (HMG-CoA) which also raises the HDL-cholesterol level by 5-10% [10]. Statins also attenuate hyperglycemia-induced oxidative stress through inhibition of vascular NADPH-oxidase [15]. However, the side effects of statins are not less and include muscle, liver damage and even neuronal toxicity [16]. Because of the side effects of statins, much research was interested in the development of plants with hypolipidemic and antidiabetic effects. The beneficial effect of is link to their composition of a wide variety of secondary metabolites such as isoflavones, phytosterols, saponins, fibers, polyphenols, tannin and flavonoids which are compounds that are attracting a lot of interest for their role in regulating blood lipid and sugar as well as the antioxidant properties of the body [13]. Several plant extracts have been experimentally tested for their potential to improve various conditions, and in many cases polyphenols and flavonoids contents play major roles in their functionality [13,15]. The present study focused on the seeds of Boscia senegalensis, a plant with potential antihypolipidemic, antihyperlipidemic and antioxidant properties which has not been investigated elsewhere.
In Tchad and in Cameroon, the village community usually use Boscia seeds extract to manage diabetes and this plant has enjoyed a great reputation in all village communities where it is found. We recently reported the optimal condition of producing Boscia extract for its antioxidant and hypoglycemic activity. However, nothing is known about the use of this plant extract against the fat accumulation, installation of obesity, oxidative stress and type II diabetes. This is important to investigate since the seeds of B. senegalensis play a big role in nutrition as a protein source, but also can be prescribed as a diet to protect against metabolic disorders [17,18]. Therefore, this piece of work was designed to investigate the protective effect of B. senegalensis seed extract against hyperlipidemia, hyperglycemia and oxidative stress in dyslipidemic rats induced by feeding high fat diet and cholesterol.
Methods
Reagents
All the chemicals were purchased from Sigma-Aldrich Chemie Gmbh (Munich, Germany); Total cholesterol and total triglyceride were estimated using diagnostic kits (Fortress, UK). HDL was determined using the MONLAB kits (Barcelona, Spain); Atorvastatine MICRO LABS LIMITED (Bengaluru, India).
Sampling and Boscia senegalensis flour production
B. senegalensis seeds were collected around the University of Ndjamena, Tchad in November 2019. The species was identified, and a voucher specimen was deposited at the Facha Veterinary and Zootechnical Laboratory (N°E 1344). Dried mature seeds were carefully cleaned and manually removed defective seeds. These dry seeds were ground into fine flour using an electric grinder (Culatti, Polymix, France) equipped with a sieve with a diameter of 800μm, then sealed in polyethylene bags and kept at ambient temperature (25±2 °C) for further analyzes.
Preparation of Boscia senegalensis extract
Preliminary studies were made in the laboratory to find different therapeutic doses on animals by studying the acute toxicity of the B. senegalensis extract on rats in vivo [18]. B. senegalensis aqueous extract was produced from the seeds flour with distilled water. The production conditions of this decoction were determined in previous studies to maximize the flour mass/water volume ratio, the content of phenolic compounds, and the DPPH scavenging activity and to minimize the glycaemia index; the extraction time, temperature and flour mass/water volume ratio were 10min, 55 °C and 3/10g/mL, respectively [18]. After incubation, the sample was centrifuged at 1500rpm for 15min at 25 °C. Supernatants were collected and packaged in a 100mL volumetric glass vessel and stored at -4 °C in the refrigerator. The extract was then lyophilized, and we obtained a powder. For extract administration, the extract lyophilized was prepared by dissolving in distilled water just prior to administration.
?Phytochemical screening
A. Tannin characterization: The plant powdered sample of 2g
was boiled in 20mL of distilled water in a water bath. The
resulted water extract was filtered through a filter paper (ϕ:
0,1mm) on a conical flask. 3 drops of 0.1 % FeCl3 was added
to the filtered sample and the brownish green or a blue-black
coloration observed, showed the presence of tannins [19].
B. Flavonoid characterization: The flavonoids of the extract
were detected using the alkaline reagent test. For this test, in
0.5mL of extract there, add 3 drops of sodium hydroxide. An
intense yellow color that disappears after adding dilute HCl
indicates the presence of flavonoids [20].
C. Polyphenol detection: To 0.5mL of the extract was added
5mL of distilled water in a test tube. A few drops of 5% ferric
chloride were added and a bluish black appeared showing the
presence of polyphenols [20].
D. Anthocyanidins detection: They were detected as previously
described [21]. In the procedure, 0.5mL of extract was added to
aqueous KOH 10% (v/v). The red color suggests the presence
of anthocyanidins.
E. Glycoside detection: To research the presence of glycoside on
the extract, 4mL of extract was dried till 2mL. To it was added
1-ml of ammonium hydroxide and shaken. The appearance of
cherished red color indicates the presence of glycosides [22].
F. Alkaloids: The alkaloids of the extract were detected by
mixing 4mL of the extract with 2mL of 1% HCl and gentle
heating. Mayer’s and Wagner’s reagent were then added to
the mixture and the turbidity of the resulting precipitate was
taken as evidence for the presence of alkaloids [22] (Parekh
and Chanda, 2008).
G. Anthraquinons assay: this was done with the potassium
hydroxide test [23]. In the test, 0.5mL of extract was added
to 0.1mL of aqueous KOH solution (10%, w/v). A red color
indicates the presence of anthraquinons.
H. Saponin assay: saponins were detected using the Froth test
[24]. 0.1mL of extract was mixed 1.5mL of distilled water in
a test tube. The solution was shaken vigorously and left for
20min. The persistence of a 1cm foam for 10min indicates the
presence of saponins.
Experimental animals and design
Albinos male Wistar rats (Rattus norvegicus) weighing between 155-200g were freshly generated from the animal house of the Faculty of Science of the University of Ngaoundere. Only male rats were used for the study in order to avoid the changes in menstrual cycle of females which may affect the results, such the excretion of estrogens known to protect the onset of obesity. In vivo tests on animals were carried out in accordance with European Union guidelines for the protection of animals (EEC Council 86/609) [25]. Animals were housed in stainless metal cages at room temperature (23±2 °C) with 12 hours of light/dark cycles and relative humidity 52%. During acclimatation, the rats had free access to tap water and a standard diet (3810kcal/kg) made up of fish flour (20%), soy oil (5%), vitamin complex (1%), mineral salts (5%), sucrose (5%), cellulose (5%) and corn flour (59%), daily.
During the experimentation, thirty normal rats were divided into 6 groups of 5 rats each. The Normal control group received standard diet and the other groups were subjected to a hyperlipidemic diet (5310Kcal/kg) consisting of fish meal (20%), soybean oil (5%), coconut oil (25%), cholesterol (1%), vitamin complex (1%), mineral salts (5%), sucrose (5%) and corn flour (38%) [26]. The hyperlipidemic diet used in this study was formulated in the past in our Laboratory and was successfully tested in inducing hyperlipidemia in rats [27]. The negative control group was fed hyperlipidemic diet with distilled water (10mL/kg) administered per OS as treatment. In the positive control group, atorvastatin replaces distilled water used in the Negative control group while in the test groups, the extracts of B. senegalensis at various doses (125, 250 and 500mg/kg) replaced water. The animals were thus treated for eight weeks, the animals were weighted every week in order to monitor the installation of overweight, while the blood was sampled from the end of the tail of each rat every week for the measurement of the fasting blood sugar. The Oral Glucose Tolerance Test (OGTT) was carried out to confirm the onset of diabetes in animals. The dose of atorvastatin 10mg/kg body weight used in the present work is based on the value in literature.
At the end of the eight weeks experiment, rats were anaesthetized using di-ethylic ether, sacrificed by cardiac puncture and blood was collected for the analysis of biochemical parameters. The liver, kidney, testicle, heart and adipose tissues were equally removed and weighed to assess the relative weight and for the production of homogenates after grinding for analysis of oxidative stress parameters.
Fasting glycaemia estimation and oral glucose tolerance test (OGTT)
At the end of at the end of each week, fasting blood sugar was taken and at the end of 8 weeks, the Oral Glucose Tolerance Test (OGTT) was carried out using “ONE TOUCH Ultra” glucometer (Lifescan, U.S.A) [28]. Blood glucose was taken by cutting the posterior end of the tail vein of the animal and a drop of blood was taken on the reactive end of a strip mounted on a glucometer for direct blood glucose reading. To evaluate the OGTT test, blood glucose levels were thus taken at 0min before administration of D-glucose (3g/kg) and then the glycaemia was measured at 30 min, 60 min and 120 min in each animal. The glucose concentrations were expressed as mg/mL blood.
Determination of biochemical parameters
Serum was collected from the clotted blood at room temperature by centrifugation at 3000rpm for 10min at 25 °C. Total cholesterol and triglyceride were estimated using diagnostic kits (Fortress, UK). HDL was determined using the MONLAB kits. VLDLC and LDLC were calculated using the Fried Ewald equation as follows: VLDLC=Serum triglyceride/5 while LDLC=Serum totalcholesterol–( VLDLC+HDLC). Transaminase ALT and AST activities were determined using the method of Reitman & Frankel [29]. Creatinine was estimated using the procedure of Bartels et al. [30]. Results were expressed in mg/dL.
Body weight, relative weight of organs and biochemical estimation in tissue homogenates
The variation of weight gain of each rat was measured weekly.
The liver, kidney, testicle, heart and abdominal fat were freed from
adhering tissues and washed with ice-cold normal saline solution
(0.9%). The relative weight of each organ was calculated as follows:
Relative weight=(organ weight)/(body weight)×100 (1)
After the relative weight was calculated, 1g tissue was
homogenized and ground in 10mL of 0.2M Tris-HCl. The homogenate
was filtered and then centrifuged at 2500rpm for 20min at 25 °C.
The supernatant obtained was used for estimation of Superoxide
Dismutase (SOD), Catalase (CAT) and Malondialdehyde (MDA) [28].
The MDA level was expressed using the Beer-Lambert formula.
Optical Density=ε.L.C. (2)
CAT and SOD activities expressed as:
A=(Total inhibition)/50 ×Fd (3)
Statistical analysis
The data are presented as mean ± SD from five animals in each group and 3 measurements per animal. All data were statistically analyzed using one-way analysis of variance (ANOVA) followed by post-hoc test (Student-Newman-Keuls) using Graph Pad Prism software version 5.03. A value of p<0.05 was considered significant statistically.
Results
Different compounds present in the Boscia senegalensis extract
Table 1:Different compounds present in the Boscia senegalensis extract.

The phytochemical studies made on the B. senegalensis seeds extract revealed the presence of numerous bioactive compounds such as: Alkaloids, saponins, tannins, glycosides, flavonoids, anthraquinones and polyphenols (Table 1).
Effect of Boscia senegalensis extract on food intake
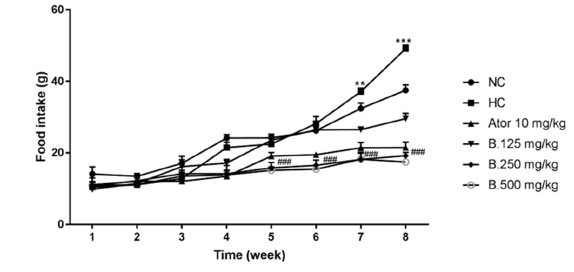
The anti-obesity effect of B. senegalensis seed extract was evaluated in rats by simultaneously administering to these animals a high-lipid diet associated with the extract of this plant for 8 weeks. Animals of negative control group that received the high fat diet with distilled water only as treatment present a significant (p<0.001) increase in polyphagia marked by excessive food intake compared to animals in the normal control group that received the normal diet and distilled water. On the other hand, for the animals’ groups which received simultaneously the hyper lipidic food and the extract of B. senegalensis (125, 250 and 500mg/kg) and those having received the reference drug (Atorvastatine 10mg/kg), the polyphagia was significantly (p<0.001) reduced compared to that of animals in the hyper lipidic control group (or negative controle group) (Figure 1).
Figure 1: Effect of Boscia senegalensis extract on food intake in hyperlipidemic diet-induced hyperlipidemia in rats during 8 weeks of treatment. Data expressed by means (X)±Standard Deviation (SD); (n=5). Significant difference; ###p˂0,001 vs Hyperlipidemic control (H. CO; ***p˂0,001: vs Normal control (N. CO). B.125mg/kg: B. senegalensis 125mg/kg; B.250mg/kg: B. senegalensis 250mg/kg; B.500mg/kg: B. senegalensis 500mg/kg; ATOR: Atorvatatine 5mg/kg.
Effect of Boscia senegalensis extract on body weight and on fasting blood glucose in hyperlipidemic diet-induced hyperlipidemia in rats during 8 weeks of treatment
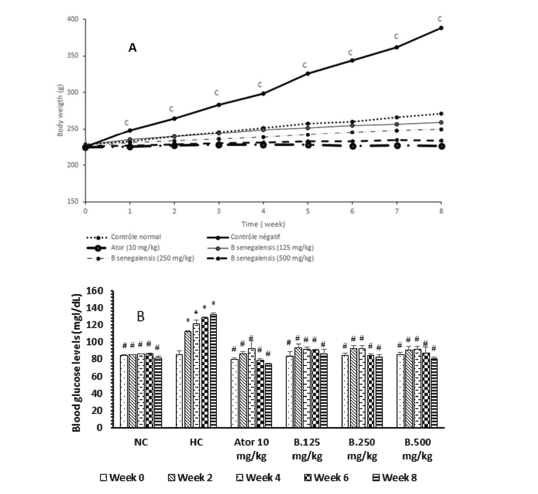
At the start of the experimentation, the mean rat weight was 225.3±0.5g in each group. During the feeding, the rat weight has varied significantly amongst groups. Rats fed normal regime (group I) showed a general fair increase in body weight, 3.1% and 19.4% at weeks 1 and 8 respectively. However, rats fed hyperlipidemic diet (group II or hyperlipidemic control group) showed at week 1, 10.4% increase in body weight and at week 8, 73.5% increase in body weight. Compared to group I. Animals of group II showed significant increase (p<0.001) in body weight 3.4 times more, suggesting the installation of obesity. When rats fed high fat were administered Boscia extract 125mg/kg, we observed an increase in weight similar to that of normal control. At 250 and 500mg/ kg of B. senegalensis extract, we no longer observed an increase, but a decrease at week 1. In this respect at 500mg/kg, the drop in weight were 2.0 times less with a percentage of 3.1% in week 1 and 7.3 times less with a percentage of 14% in week 8. In addition, Atorvastatin had an effect on reducing body weight similar to that of B. senegalensis extract at 250mg/kg (Figure 2A).
At the beginning of the experiment the mean fasting blood glucose levels (83.7±1.9mg/mL) were not significantly different. In rat fed normal standard diet, the blood glucose was constant at 84.4±1.9mg/mL during the 7 weeks but has significantly dropped at the week 8 to 69.8±1.9mg/dL. When rats were submitted to the hyperlipidemic diet for 8 weeks, we observed that fasting blood sugar has increased and rose to 139.6±0.9mg/dL at the end of week 2 and 149.2±1.9mg/dL at the end of week 8. This result demonstrates the installation of hyperglycemia and thus of diabetes and it justifies the use of hyperlipidemic diet to induct metabolic syndrome in animals. In rats submitted to hyperlipidemic diet, the concomitant consumption of Boscia extract prevented fasting hyper glycaemia. In fact, in the group of animal administered 500mg/kg of B. senegalensis extract, the blood sugar level fell compared to that of the negative control animals and went from 91.0±1.0mg/dL to 81.0±1.0mg/dL respectively at week 0 and at the end of week 8. This result shows that B. senegalensis extract prevents the increase in blood sugar. Atorvastatin at 10mg/kg used as drug control against hyperlipidemia also had effects similar to extract, limiting the increase in blood sugar which went from 92.4±8.7mg/dL at week 4, followed by a decrease to 74.4±1.1mg/dL at week 8 (Figure 2B).
Figure 2:Effect of Boscia senegalensis extract on body weight (A) and on fasting blood glucose (B) in hyperlipidemic diet-induced hyperlipidemia in rats during 8 weeks of treatment. Data expressed by means (X)±Standard Deviation (SD); (n=5). Significant difference; #p˂0,001 vs Hyperlipidemic control (H. CO; cp˂0,001: vs Normal control (N. CO). B.125mg/kg: B. senegalensis 125mg/kg; B.250mg/kg: B. senegalensis 250mg/kg; B.500mg/kg: B. senegalensis 500mg/kg; Ator: Atorvatatine 5mg/kg.
Effect of Boscia senegalensis extract on relative organ weight
Globally amongst the organ studied, the liver has the higher relative weight, followed by abdominal fat. In animals fed hyperlipidemic diet, all the organs increased in weight, abdominal fat increased and went from to 3.38±2.94g/100g compared to 1.26±0.06g/100g observed to the normal animals. The percentage of increase in abdominal fat is 168%, while testicle had the lowest increase 41.4%. In hyperlipidemic animals, administered Boscia extract, the increase in organ weight was very low, and this varied with the dose of Boscia extract. A 12% decrease in heart relative weight was observed at both doses 250 and 500mg/kg. At dose 500mg/kg, a decrease of 5% and 2% were equally observed respectively in liver and in testicle (Table 2).
Table 2:Relative weight of kidney, heart, testicle, liver and abdominal fat in hyperlipidemic rats after 8 weeks treatment with Boscia senegalensis extract. Values represent means±SD; (n=5). Significant difference: *p˂0. 05; **p˂0, 01 vs Normal control (N. CO); #p˂0.05; ##p˂0.01 vs Hyperlipidemic control (H. CO) Ator: Atorvastatine. B.125mg/kg: B. senegalensis 125mg/kg; B.250mg/kg: B. senegalensis 250mg/kg; B.500 mg/kg: B. senegalensis 500mg/kg.

Effect of Boscia senegalensis extract on blood glucose concentration and on biochemical parameters in hyperlipidemic diet-induced hyperlipidemia in rats after 8 weeks of treatment
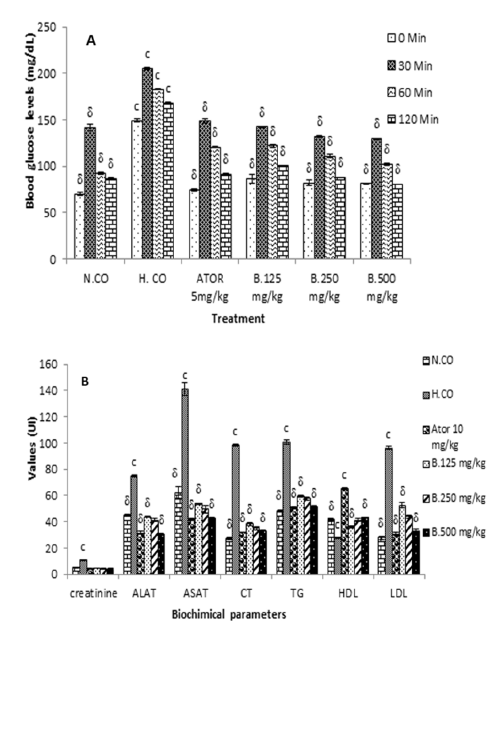
At the end of the 8th week, the OGTT test was carried out to confirm the onset of diabetes in the rats of the diabetic control group and the protection of the animals having received the Boscia extract against the onset of diabetes (Figure 3A). Significant differences were observed in blood sugar levels from 30 to 120 minutes after administration of D-glucose. At 120 minutes the blood sugar value had significantly (p<0.01) decreased in the group of animals having received Boscia extract. At 120min, glycaemia were 86.2±0.8; 168.4±4.7; 91.4±1.1; 87.6±0.8 and 80.8±0.8 respectively for normal control (N.Co), hyperlipidemic control H.Co), positive control (Ator) and Boscia extra (B. 250 and 500mg/kg). The maximum reduction in blood glucose was obtained with the dose 500mg/kg of body weight at the 120th minute after administration of D-glucose of the order of 100%.
After administration normal diet (to the normal group animals) and hyperlipidemic diet (to hyperlipidemic and greatest animals’ groups) feeding until 8 weeks, the values of biochemical parameters significantly increased in animals in the negative control group compared to animals in the normal control. these values are 10.8±0.41mg/dL; 74.8±0.72U/L and 141.2±4.58 U/L respectively for creatinine; Alat and ASAT. However, the simultaneous administration of Boscia extract (500mg/kg) and the hyper lipidic food prevents the increase in the rate of creatinine (3.72±0.44mg/ dL); Alat (30.26±0.90U/L) and ASAT (42.58.26±0.62U/L). Likewise in the animals of the hyper lipidic control group, the values of the lipid profile parameters were significantly increased compared to those of animals in the normal control group. these values are 96.2±0.76mg/dL; 101±1.58mg/dL and 96.2±1.30mg/dL respectively for total cholesterol, triglycerides and LDL cholesterol. However, the simultaneous administration of Boscia extract (500mg/kg) and the hyper lipidic food prevents the increase in the rate of total cholesterol (32.52±0.9), triglycerides (51±0.83) and LDL cholesterol (32.6±1.51). Compared to hyperlipidemic diet the extract at 500mg/kg increase significant (p<0.001). In animals in the negative control group the percent increase in LDL cholesterol was 33% while the ALAT percent increase varied from 66% and the one of total cholesterol was 150% when compared to those of animals treated with 500mg/kg of Boscia extract. The hyperlipidemic diet induced in rats within 8 weeks experimentation hyperliidemia characterized by hypertriglyceridemia, hypercholesterolemia and hypercreatinemia. Administration of Boscia extract also significantly protects decrease in HDL during feeding of hyperlipidemic diet, The effect of Atorvastatin was in general similar to that of Boscia 500mg/kg, higher (Figure 3B).
Figure 3:Effect of Boscia senegalensis extract on oral glucose tolerance test (A) and on Biochemical parameters (B) in hyperlipidemic diet-induced hyperlipidemia in rats after 8 weeks of treatment. Data expressed by means (X)±Standard Deviation (SD); (n=5). Significant difference: δp˂0,001 vs hyperlipidemic control (H. CO); cp˂0,001: vs Normal control (N. CO). B.125mg/kg: B. senegalensis 125mg/kg; B.250mg/kg: B. senegalensis 250mg/kg; B.500mg/kg: B. senegalensis 500mg/kg; ATOR: Atorvatatine 5mg/kg.
Effect of Boscia senegalensis extract on tissue antioxidant parameters in hyperlipidemic diet-induced hyperlipidemia in rats after 8 weeks of treatment
In normal control group the levels of SOD, CAT and MDA were 6.6-9.7μU/mg, 39.2-44.0U/mg et1.1-13μM in plasma, liver and kidney respectively. Irrespective of the diet and drug administration regimes, stress parameters were lower in the blood and higher in the kidney. We notice highly significant elevations (p<0.001) of MDA and reduction of SOD and CAT in organs of hyperlipidemic control group when compared to the normal control group. The hyperlipidemic diet was then efficient to induce oxidative stress in animals through reduction of antioxidant enzymes SOD and CAT and increase of marker of fat oxidation MDA. Compared to rats fed a normal diet, the decrease in SOD and CAT were respectively 46-49% and 14-19% while the increase in MDA was 102-172%. The simultaneous administration of the hyper lipidic diet with Boscia extract protected the organs against changes induced by the hyperlipidemic diet. Comparatively to rats fed only with hyperlipidemic diet, SOD concentration was 1.6-time times higher while MDA concentration was 2 times lower in rats treated with Boscia at 500mg/kg. Indeed, Boscia extract exerted an antioxidant activity which was also found to be dose dependent. The antioxidant power of Boscia extract was higher on the rat which received the extract of Boscia at 500mg/kg had elevated catalase concentration in liver compared to normal control group. Atorvastatin 10mg/kg had similar activity on oxidative stress parameters in response in between Boscia extract at 250 and 500mg/kg (Table 3).
Table 3:Malondialdehyde (MDA), Catalase (CAT) and Superoxide dismutase (SOD) in Liver, kidney and serum. Values represent means±SD; n=5; significant difference *p˂0. 05; **p˂0, 01; ***p˂0, 001 vs Normal control (N.CO) group; #p˂0.05; ##p˂0.01; ###p˂0.001 vs hyperlipidemic control (H.CO). Ator: Atorvastatine 10mg/kg; B.125mg/kg: B. senegalensis 125mg/kg; B.250mg/kg: B. senegalensis 250mg/kg; B.500mg/kg: B. senegalensis 500mg/kg; ATOR: Atorvatatine 5mg/kg.

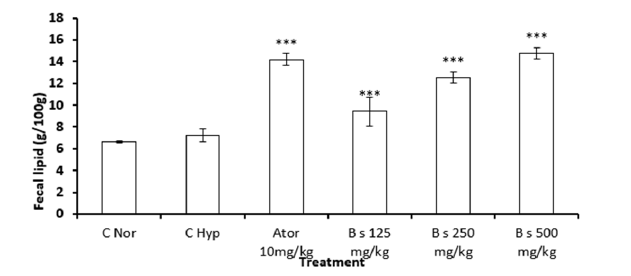
Effect of Boscia senegalensis extract on fecal fat elimination in hyperlipidemic diet-induced hyperlipidemia in rats during 8 weeks of treatment
In rats fed the normal diet, the rate of fecal fat eliminated was 6.63±0.07%. In animals fed a high-fat diet and treated with distilled water, no significant differences were observed in the elimination of fat in the stool compared to animals in the normal group. In rats fed a hyperlipidemic diet and treated with Boscia extract, excreted fecal fat was significantly (p<0.001) increased. Fecal fat levels in rats treated with Boscia at 125, 250 and 500mg/kg were 9.2±1.3%, 12.5±0.5% and 14.8±0.5%, respectively. compared to the normal diet. We observed that the percentage of elimination of fecal fats increased by 37% and 120% respectively at doses 125 and 500mg/ kg of Boscia extract respectively and that atorvastatin caused the elimination of 10% of these fats in feces. Atorvastatin had similar elimination of fecal fat as Boscia extract at a dose of 500mg/kg (Figure 4).
Figure 4:Effect of Boscia senegalensis extract on fecal lipid level in hyperlipidemic diet-induced hyperlipidemia in rats. Data expressed by means (X)±Standard Deviation (SD); (n=5). Significant difference: ***p˂0.001 vs Normal control (N.CO). H.CO: Hyperlipidemic Control; B.125mg/kg: B. senegalensis 125mg/kg; B.250mg/kg: B. senegalensis 250mg/kg; B.500mg/kg: B.senegalensis 500mg/kg; Ator: Atorvatatine 5mg/kg.
Discussion
B. senegalensis is a plant used empirically by traditional healers to treat diabetes and obesity. The seeds extract contains many bioactive components which may explain their efficacy in the treatment of several metabolic disorders. Polyphenols, flavonoids, tannins, saponins and anthocyanidins identify in the seed extract belong to families of hypoglycemic and hypolipidemic compounds fully investigated in the literature [31-33]. The present work failed to quantify this compound and this needs to be investigated in the future.
The polyphagia observed in the animals of the hyperlipidemic control group would be the cause of the overweight observed and even of the dyslipidemia observed in these animals [34]. It has been reported that hyperlipidemic diet can induce lipid accumulation and promote hyperlipidemia and hyperglycemia in rats by a nutritional intervention [35,36]. According to some authors, hyperlipidemic diets can be used to generate a valid rodent model for the analysis of the pathophysiology of dyslipidemia and diabetes [37,38]. In this study, the feeding with high fat-cholesterol diet (hyperlipidemic diet) for 8 weeks successfully induced fat accumulation, hyperlipidemia, hyperglycemia and oxidative stress.
In this study, dyslipidemia was induced in rats by feeding animal whit hyper lipidic diet (high fat diet) and B. senegalensis decoction was administrated simultaneously as prevention against hyperlipidemia until 8 weeks. This high fat diet induced the perturbation in blood glucose and lipidic parameters. The oral administration of B. senegalensis decoction at the doses of 125mg/ kg, 250mg/kg and 500mg/kg of body weight and Atorvastatin at 10mg/kg reduced simultaneously the level of blood glucose and the lipid parameter level. In the present study, B. senegalensis significantly prevents the increase of blood glucose induced by the high fat diet when compared to hyperlipidemic control group at all doses during all the experimental periods. This result suggests that the aqueous extract of B. senegalensis possessed antihyperglycemic and antihyperlipidemic effects. This result could be explained by the stimulation of insulin secretion and or glucose uptake in muscle and adipose tissue [39]. The antihyperglycemic effect of B. senegalensis decoction was confirmed with OGTT test previously rated. The boscia extract significantly prevented the increase of glycemia after glucose load. These results at the dose of 500mg/kg are similar to those of the standard oral antihyperlipidemic drug as Atorvastatin (10mg/kg). This drug is a selective and competitive inhibitor of HMG-CoA reductase. This enzyme is responsible for the control of the rate of biotransformation of 3- hydroxyl- 3- methylglutaryl- coenzyme A into mevalonate. Atorvastatin reduced at 30% the relative risk of diabetes manifestation [40]. In addition, a Canadian retrospective study proved a relationship between statine and time of insulin passage among patient having diabetes [41]. B. senegalensis could similarly stimulate the insulin released.
It has been well established that nutrition plays an important role in the etiology of body weight and hyperlipidemic. For body weight, several animal and human studies have confirmed the effect of saturated fatty acids and cholesterol on the increase in body weight of animals. This result showed that the body weight of hyperlipidemic control group fed with the high fat diet significantly increased when compared to normal control group fed with the standard diet. The treatment with B. senegalensis extracts respectively at doses 125, 250 and 500mg/kg and Atorvastatin at 10mg/kg has significantly reduced the body weight of rats when compared to hyperlipidemic control group feeding with hyperlipidemic diet at the end of week 8. The relative weight of kidney and heart increases with hyperlipidemy and the administration of the high fat diet simultaneously with extract of boscia prevents the increase in the weight [42]. These results are similar to those found in literature. In the same way, the kidney and the liver weight where significantly (p<0.05) increased in hyperlipidemic control group compared to normal control, but the heart and abdominal fat weight where significantly (p<0.05) increased on hyperlipidemic control group compared to normal control group. The increase of body weight and kidney suggested the installation of dyslipidemic with the high fat diet induced hyperlipidemic [43]. Our results are also similar to those which showed that rats fed with a diet supplemented with cholesterol, Cholic acid and coconut oil for 30 days served as the experimental model of hyperlipidemic [42].
The increase in the body weight and fat deposition in the tissue is the real indicators for the gradual progress of dyslipidemia and obesity. As the animals were fed with hyperlipidemic diet, there was an increase in adiposity, which in turn increased the fat cell mass. Thus, there was an overall increase in body weight. The increased body weight found in hyperlipidemic diet rats might be due to the consumption of a diet rich in energy, in the form of saturated fats (lard) and its deposition in various body fat pads and decreased energy expenditure as compared to standard diet-fed [44].
However, a significant decrease in body weight and fat mass (interscapular as well as epididymal) was observed in B. senegalensis treated rats. It is well known that dyslipidemia is a highly predisposing condition for arteriosclerosis and other cardiovascular diseases [45]. It is well documented that elevated total cholesterol and LDL-cholesterol levels promote atherosclerosis and cardiovascular complications [27]. Hyperlidemic diet feeding in rats caused a significant increase in the circulating total cholesterol, LDL-cholesterol and simultaneously increased immediately the reduction of HDL-cholesterol [46,47]. These results have clearly established a correlation between dietary lipids and serum lipid profile [46,47]. Simultaneous administration of B. senegalensis extract and hyperlipidemic caused a significant decrease in serum total cholesterol, LDL-cholesterol, suggesting beneficial modulator influence on cholesterol metabolism. Elevated serum triglyceride is considered as an independent risk factor for cardiovascular disease [48]. A significant decline in the serum triglycerides level observed in B. senegalensis extract and Atorvastatin treated rats supports the cardiovascular protective influence. The cholesterol lowering effect of the Atorvastatin is possibly associated with a decrease in intestinal absorption of cholesterol resulting in an increase in fecal excretion of neutral lipids [49].
Feeding with hyperlipidemic diet induced a significant increase of AST, ALT and creatinine. The administration of the plant decoction simultaneously with the hyperlipic diet significantly prevented the rise of those parameters suggesting a protective effect of B. senegalensis decoction in liver, kidney and serum function. Because of the misbalanced oxidative/anti oxidative system in hyperlipidemic and its involvement in the mechanism of various pathological complications, the effect of the aqueous extract of plant on the oxidative stress [49]. We had found that hyperliplipidic diet increased both the lipid peroxidation to increase the rate of MDA and decrease the antioxidant enzyme (SOD and CAT) activities, in hyperlipidemic control rats. The authors delineated that the increase in antioxidant enzyme activities on the animals could primarily be a response to the hyperlipidemic state [50,51]. Interestingly, the administered of B. senegalensis extract associated whit hyperlipidic diet potentially prevented the increase in the rate of the liver, kidney and serum MDA parameter. The antioxidant defenses consist of low molecular mass antioxidants, intracellular enzymes, sequestration of transition metal ions and repair mechanisms [52]. Particularly important low molecular mass antioxidants are glutathione, vitamin E, ubiquinone, -carotene, vitamins C and A. GSH and vitamin C strongly prevent the lipid peroxidation and DNA damages induced by the hydroxyl radical [53].
Currently used drugs against hyperlipidemic and diabetes are essentially focused on controlling and lowering lipid profile and blood glucose to a normal level. Some undesirable side-effects such as hypoglyceamia shock, lactic acid intoxication and gastrointestinal upset appear after patients take these medicines [54]. To disclose the wonder, alternative medicines look like an adequate solution. The reported results, herein, suggest that B. senegalensis aqueous extract is able to re-establish both the blood glucose level, the lipid profile and the oxidative stress status, which are determinants of hyperlipidemic secondary complications as diabetes, arterial hypertension obesity. Previous research shows that B. senegalensis decoction extract possesses the phenolics compounds that can free radical scavenging (DPPH). La glucocapparin, are the active molecule in B. senegalensis decoction [18,55]. It comes from this study that phenols may play a significant role either alone or in interaction with other hydrophilic compounds on the therapeutic effect of hyperlipidemic.
Conclusion
The present study showed that the decoction of B. senegalensis seeds has antihyperglycemic, antihyperlipidemic potency and also acts as a powerful oxidative antistress in animals whose dyslipidemia was experimentally induced with a high-fat diet. Therefore, these results lend credence to the traditional use of B. senegalensis seed decoction by traditional healers to treat diabetes and related diseases.
Acknowledgement
Our thanks go to Pr Mapongmetsem of the Biodiversity Laboratory of University of Ngaoundere for his help in identifying the plant.
References
- Lois K, Young J, Kumar S (2008) Obesity; Epiphenomenon or cause of metabolic syndrome? Int J Clin Pract 62(6): 932-938.
- Kannel WB (1993) Hypertension as a risk factor factor for cardiac events epidemiologic results of long-term studies. J Cardiovasc Pharmacol 21(Suppl 2): S27-S37.
- Andreassi MG, Barale R, Iozzo P, Picano E (2011) The association of micronucleus frequency with obesity, diabetes and cardiovascular disease. Mutagenesis 26(1): 77-83.
- Astrup A (2001) Healthy lifestyles in Europe: prevention of obesity and type II diabetes by diet and physical activity. Public Health Nutr 4(2B): 499-515.
- Žáček P, Bukowski M, Mehus A, Johnson LA, Zeng H, et al. (2019) Dietary saturated fatty acid type impacts obesity-induced metabolic dysfunction and plasma lipidomic signatures in mice. J Nutr Biochem 64: 32-44.
- Yang JY, Lee SJ, Park HW, Cha YS (2006) Effect of genistein with carnitine administration on lipid parameters and obesity in C57B1/6J mice fed a high-fat diet. J Med Food 9(4): 459-467.
- Thiombiano LP, Mbaye A, Sarr SA, Ngaide AA, Kane Ab, et al. (2016) Prevalence of dyslipidemia in the rural population of Guéoul (Senegal). Ann Cardiol Angeiol (Paris) 65(2): 77-80.
- Arlotto E, Felicé MP, Favre C, Bun H, Cornet M (2008) Interest of the entry biological assessment in the screening of comorbidities in patients hospitalized in psychiatry. Brain 34(1): 61-65.
- Béliard S, Valéro R (2020) Guidance on prescribing statins. Nutr Clin Metab 34(3): 223-226.
- Marie I, Boyer O (2013) Statin-related inflammatory myopathy. Rev Med Interne 34: 333-336.
- Maddux BA, See W, Lawrence JC, Goldfine AL, Goldfine ID, et al. (2001) Protection against oxidative stress-induced insulin resistance in rat l6 muscle cells by micromolar concentrations of α-lipoic acid. Diabetes 50(2): 404-410.
- Bloch Damti A, Bashan N (2005) Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal 7(11-12): 1553-1567.
- Shulman GI (2020) Cellular mechanisms of insulin resistance. J Clin Invest 106(2): 171-176.
- Yu C, Chen Y, Cline GW, Zhang D, Zong H (2002) Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277(52): 50230-50236
- Ray KK, Cannon CP (2005) The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol 46(8): 1426-1433.
- Komajda M, Commission D, Maladies IV (2018) Efficacy and side effects of statin therapy: a reappraisal. Bull Acad Natl Med 202(5-6): 817-835.
- Kim TR, Pastuszyn A, Vanderjagt DJ, Glew RS, Millson M, et al. (1997) The nutritional composition of seeds from Boscia senegalensis (Dilo) from the Republic of Niger. J Food Compos Anal 10(1): 73-81
- Dongmo F, Sokeng SD, Njintang YN (2017) Aqueous extraction optimization of the antioxidant and antihyperglycemic components of boscia senegalensis using central composite design methodology. HSOA J Food Sci Nutr 3: 1-7.
- Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 4(7): 685-688
- Khanam Z, Wen CS, Bhat IUH (2015) Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat ali). J King Saud Univ Sci 27(1): 23-30.
- Sayout A, Bahi F, Ouknin M, Arjouni Y, Majidi L, et al. (2015) Phytochemical screening and antioxidant activity of four Moroccan Thymus species: T leptobotrys Murb, T pallidus Batt, T broussonetti Boiss and T Maroccanus Ball. Arab J Med Aromat Plants pp. 117-128.
- Parekh J, Chanda S (2008) Antibacterial and phytochemical screening of some plants from western region of India. Plant Archives 8(2): 657-662.
- Dohou R, Yamni K, Tahrouch S, Hassani LI, Badoc A (2003) Phytochemical screening of an Ibero-Moroccan endemic, Thymelaea lythroides. Bull Soc Pharm Bord 142: 61-78.
- Sabri FZ, Belarbi M, Sabri S, MS Alsayadi M (2012) Phytochemical screening and identification of some compounds from mallow. J Nat Prod Plant Resour 2(4): 512-516.
- Smith J, Van den Broek F, Martorel JC, Hackbarth H, Ruksenas O (2007) FELASA Working Grp, and “principles and practice in ethical review of animal experiments across Europe: Summary of the report of a Felasa working group on ethical evaluation of animal experiments. Lab Anim 41:143-160.
- Hamlat N, Neggazi S, Benazzoug Y, Kacimi G, Chaîb S (2008) Regime hyperlipidique et procesos atherosclereux chez Rattus norvegicus. Sci Technol 27: 49-56.
- Ngatchic JTM, Njintang NY, Bernard C, Oben J, Mbofung CM (2016) Lipid-lowering properties of protein-rich mucuna product. Nutrir 41(2): 1-10.
- Israni DA, Patel KV, Gandhi TR (2010) Anti-hyperlipidemic activity of aqueous extract of terminalia chebula & gaumutra in high cholesterol diet fed rats. Int J Pharm Sci 1: 48-59.
- Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxlacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1): 56-63.
- Bartels H, Böhmer M, Heierli C (1972) Serum creatinine determination without deproteinization. Clin Chim Acta 37: 193-197.
- Dadu KB, Sumera Q, Allah BG (2014) An overview of available hypoglycemic triterpenoîde and saponins to cure diabetes mellitus. International Journal Advancement in Life Sciences 1(3): 119-128
- Khera N, Bhatia A (2014) Medical plants as natural antidiabetic agents. International Journal of Pharmaceutical Sciences and Research 5(3): 713-729
- Nayak BS, Marshall RJ, Milne D, Kanhai J, Kantikar SM, et al. (2011) Hypoglycemic activity of Chrysobalanus iaco (Fat-pork) fruit extract in diabetes induced rats. Asian Journal of Pharmaceutical and Biological Research 1(4): 512-517.
- Ngatchic Metsagang JT, Njintang Yanou N, Bernard C, Oben J, Mbofung CM (2016) Lipid-lowering properties of protein-rich mucuna product. Nutrire 41:2
- Cao JJ, Sun L, Gao H (2010) Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci 1192: 292-297.
- Shimamura Y, Yoda M, Sakakibara H, Matsunaga K, Masuda S (2013) Pu-erh tea suppresses diet-induced body fat accumulation in C57BL/6J mice by down-regulating SREBP-1c and related molecules. Biosci Biotechnol Biochem 77(7): 1455-1460.
- Brooks SPJ, Lampi BJ (1999) Effect of dietary fat on whole body fatty acid synthesis in weanling rats. J Nutr Biochem 10(5): 291-298.
- Estadella D, Oyama LM, Dâmaso AR, Ribeiro EB, Oller DO, et al. (2004) Effect of palatable hyperlipidic diet on lipid metabolism of sedentary and exercised rats. Nutrition 20(2): 218-224.
- Dimitriadis G, Mitron P, Lambadiari V, Maratou E, Raptis SA (2011) Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract 93(1): 52-59.
- Freeman DJ, Norrie J, Sattar N, Neely RDG, Cobbe SM, et al. (2001) Pravastatin and the development of diabetes mellitus: Evidence for a protective treatment effect in the West of Scotland coronary prevention study. Circulation103(3): 357-362.
- Ishii Y, Ohta T, Sasase T, Morinaga H, Hata T (2010) A high-fat diet inhibits the progression of diabetes mellitus in type 2 diabetic rats. Nutr. Res 30(7): 483-491.
- Patil MN, Kagathara VG, Harle UN, Pujari RR, Ingawale DK (2010) Effect of polyherbal formulation in obesity associated diabetes. Int J Pharm Pharm Sci 2(3): 180-186.
- Zulet MA, Barber A, Garcin H, Higueret P, Martínez JA (1999) lterations in carbohydrate and lipid metabolism induced by a diet rich in coconut oil and cholesterol in a rat model. J Am Coll Nutr 18(1): 36-42.
- Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW (1986) Fat feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. Am J Physiol Endocrinol Metab 251(5): 576-583
- Levine GN, Keaney JF, Vita JA (1995) Cholesterol reduction in cardiovascular disease clinical benefits and possible Mechanisms. N Engl J Med 332(8): 512-521.
- Lewis GF, Carpentier A, Adeli K, Giacca A (2002) Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 23(2): 201-229.
- Yang SF, Tzang BS, Yang KT, Hsiao YC, Chang YY, et al (2010) Taurine alleviates dyslipidemia and liver damage induced by a high-fat/cholesterol-dietary habit. Food Chem 120(1): 156-162.
- Bray GA, Bouchard C (2014) Handbook of Obesity. Volume 2: Clinical Applications, Fourth Edition 2: 528
- Kyznetsova MY, Makieieva OM, Lavrovska DO, Tymoshenko MO, Sheverova DP, et al. (2015) Effect of aqueous extract from Phaseolus vulgaris pods on lipid peroxidation and antioxidant enzymes activity in the liver and kidney of diabetic rats. J Appl Pharm Sci 5(5): 1-6.
- Adekunle AS, Adedeji AL, Oyewo EO, Adedosu OT, Omotoso AT (2013) Hyperlipidemia induced by atherogenic diet enhanced oxidative stress in the kidney and inflamatory responses: An In-Vivo Asian J Nat Appl Sci 2(1): 82-93.
- Evans P, Halliwell B (2001) Micronutrients: oxidant/antioxidant status. Br J Nutr 85(2): 67-74
- Tan BL, Norhaizan ME, Liew W (2018) Review article nutrients and oxidative stress: Friend or foe? Oxid Med Cell Longev.
- Li WL, Zheng HC, Bukuru J, De Kimpe N (2004) Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 92(1): 1-21.
- Patel A (2004) Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation 110(17): 2678-2686.
- Sakine MNA, Mahmout Y, Dijoux-Franca MG, Gbenou J, Moudachirou, M (2012) In vitro anti-hyperglycaemic effect of glucocapparin isolated from the seeds of Boscia senegalensis (Pers.) Lam ex Poiret. African J Biotechnol 11(23): 6345-6349.
© 2023 Faustin Dongmo. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






