- Submissions

Full Text
Gerontology & Geriatrics Studies
Some Observations on Visual Attention Style Through the Administration of two Clinical Tests on Subjects with Parkinson’s Disease
Francesco Benso1, Eleonora Ardu2*, Gabriele Volpara3, Eva Benso2, Fabrizio Bracco4, Giulia Iacoponi2 and Carlo Serrati5
1University of Trento, Italy
2ANCCRI Associazione Neuroscienze Cognitive Clinica Ricerca Intervento, Italy
3ICoN Cognitive Neuroscience center, Institute for Advanced Studies, Italy
4Department of educational sciences, University of Genoa, Italy
5Department of of Neurology, Imperia Hospital, Italy
*Corresponding author:Eleonora Ardu, ANCCRI Associazione Neuroscienze Cognitive Clinica Ricerca Intervento, Italy
Submission: June 21, 2023; Published: July 10, 2023

ISSN 2578-0093Volume8 Issue4
Abstract
Backgrounds and aims: Visual misperceptions due to dopamine depletion could take place at an early stage of the Parkinson’s Disease (PD). However, there is evidence in literature of inconsistent results about visual attention skills of parkinsonian patients undergoing medical treatment. Some authors found some difficulties in PD engaged in a visual attention task of salient stimuli with distributed attention, while it is similar when they have to search for targets among distractors (with feature conjunctions). Other authors, however, do not find evidence of impairments in the visual attention task. We will try to clarify some of these aspects with the results of our research.
Method: We conducted a preliminary experiment to assess whether patients could have a better performance in a “Pop Out” visual search when set in a “Top Down” mode. We then conducted Study 1, in which we administered a cancellation test that separates the motor from the visual attention component. In Study 2, participants performed the Navon test (1977) in order to investigate visual attention style.
Result: Results show that patients with PD have hesitations in the cancellation test mainly due to the motor impairment, not in the serial visual attention. In the Navon task, we reported a narrow and closed focusing style.
Discussion:Let’s assume that these effects could be due either to the low contrast sensitivity in their retinal periphery, or to the excessive protective function of peripheral attentional filters that try to compensate the deteriorated central filters, partly located in basal ganglia. Further investigations will need to follow.
Keywords:Parkinson; Visual attention; Cancellation test; Navon test
Introduction
Several studies on Parkinson’s Disease (PD) report, among secondary symptoms, visual impairments mainly due to dopamine depletion. Patients have a greater latency in visual evoked potentials (VEPs;) [1,2], abnormal electroretinogram (pERG) [3,4], and a reduced contrast sensitivity, mediated by retinal deficits [5], which impairs the discrimination of fine details [6]. The functional neuroanatomical areas supporting these effects are partially unknown. However, taking into account the peculiar VEPs latencies, several authors argue that these dysfunctions could have a dopaminergic origin at the retinal level [3,7]. Bodis-Wollner et al. [7] claimed that the interplexiform amacrine cells of the retina could be involved in visual dysfunction of PD patients. However, there is also evidence of the dopaminergic innervation of visual pathways at the level of the geniculate nucleus [8] and of the visual cortex [9]. Several studies demonstrated that l-dopa (levodopa) and dopamine agonists have positive effects both on VEPs and ERP [10] and can reduce the impairments observed on animals treated with MPTP (1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine)a [3]. Visual perception, amplitude and latency of ERG and VEP improve when the patients are administered with l-dopa or dopamine agonists and enter the so-called “On” phase, where just some of the PD symptoms are present. While, when the levodopa effect is over, patients enter the “Off” phase and the parameters significantly worsen. Several studies investigated the visual attention style and the kind of visual attention of patients with PD undergoing medical treatment.
The attentional filters
Attention has been interpreted as a filter necessary to reduce the amount of irrelevant information since 1950’s. A theoretical and experimental debate burst around which stage of perceptual processing should be affected by the attentional filter. In other words, researchers disputed whether irrelevant stimuli were selected at the early stage of the peripheral sensory input [11,12], or later on, at the higher cognitive processes stage or at the output selection stage [13-16]. After an accurate meta-analysis, Lavie & Tsall [17] concluded that the evidence for either the early or the late selection stage hypothesis depends on the kind of task and, therefore, the perceptual load imposed by it. In high perceptual load tasks, distractors are isolated and filtered at an early stage because of a lack of resources. While, in low perceptual load tasks, irrelevant stimuli are filtered at a later stage (the so-called, cognitive stage) and are, therefore, more deeply processed. Consequently, there could be evidence supporting both the theories, since the filter could take place at an early or late stage.
The peripheral early filter could passively select irrelevant information if the task imposes a high perceptual load. In this case, the resources are completely devoted to the stimulus discrimination and this prevents the processing of distractors. The late “Cognitive” selection is located at a more central stage (working memory) and could manage the irrelevant and incoherent stimuli that are processed beyond the “Perceptual” selection stage. This is what happens, for instance, in the Stroop effectb or in the Navon effect, since these are tasks with a low perceptual load [18]. In such a case, the selection is late and it directly depends on the control system. As a consequence, the response activated by the distractor is hindered or more resources are devoted to the primary task.
Visual attention in patients with parkinson’s disease
Figure 1:The time period of the findings in the literature, the collection period and the age group of the study population.

There are contrasting evidences in literature concerning visual attention style of PD patients. Troscianko & Calvert [19] and Weinstein et al. [20] argue that the visual attention of PD patients in a distributed attention condition increases according with the number of distractors, thus not taking advantage of the “Pop- Out” effect. However, Berry [21] did not find this evidence, since PD patients (except those with “Frontal” comorbidities) did not differ from the control group in terms of search time in “Pop-Out” conditions, where the slope of the RT line did not increasec. Lieb et al. [22], on the other hand, found evidence of preattentive processes problems in patients with PD and this could be due to difficulties in terms of distributed attention [23]. Furthermore, Lieb et al. [22] noticed that PD patients have a higher threshold for contrast sensitivity (when targets and distractors could be confused), as already demonstrated by Ikeda et al. [4], Masson et al. [6] & Harris et al. [24] argue that the lower contrast sensitivity of PD patients is located in the retinal periphery, and it could be due to a lack of dopamine of amacrine cells.
Horowitz et al. [25] reconsider the various interpretations taking into account the role of higher-ordered intervening variables in visual attention tasks. They argue that the low salience of a degraded stimulus (by a Gaussian noise) and the unpredictability of stimuli characteristics (as in Lieb et al. [22]) could be better explanations for PD patients difficulties in visual attention tasks. The authors did not find evidence of RT differences between PD patients and the control group in a visual attention task with salient and predictable stimuli. In other words, patients with PD have problems when the targets differ from the distractors by just one feature (singleton) that can vary unpredictably across trials. This is a bottom-up condition, where attention is captured by the target distinctiveness and the task will get more difficult as the target saliency will decrease. In addition, Horowitz et al. [25] isolate the visual attention time from the motor response decision stage. The visual attention time can be derived from the slope of the line in relation with the distractors, while the motor response is represented by the intercept of the line on the y-axis. The authors conclude that the delay of PD patients is mainly due to hesitations in the motor responsed.
Clinical trials to assess selective attention
The clinical assessment of selective attention is usually performed with “Paper-and-Pencil” visual attention tasks where observers must cancel as soon as possible targets embedded among distractors. Literature is rich of examples of this kind of cancellation tasks, where observers must cancel in a given time the highest number of targets like letters [26], numbers [27], and simple figures [28]. In this paradigm observers know in advance the target stimulus and, if the perceptual contrast with distractors is sufficiently large, PD patients should perform as well as the control group [25]. However, we could hypothesize that PD patients performance could be impaired by the higher motor load of cancellation tasks in comparison with traditional visual attention paradigms, where participants must simply press a key. Therefore, the performance in cancellation tasks could be mainly due not to the selective attention necessary for a rapid visual attention, but to the motor component (which is already significant in paradigms based on key press, as demonstrated by Horowitz et al. [25]). Therefore, in clinical diagnostics, a delay in cancellation tasks is often improperly considered as a selective attention impairment.
Given the uncertain evidences found in literature concerning visual attention efficiency of PD patients, we aim at investigating their attentive focusing style. We suspect that when patients have difficulties with “Pop-Out” targets [19-22], this could be due to a focused style, not distributed over the entire display, which is not captured by the singleton. On the other hand, when they do not have problems in detecting “Pop-Out” stimuli [21], this could be due to a well adapted and distributed focus of attention all over the display, or to a focused attention which is able to early detect the most salient target (according to the filter model theory, by Di Lollo et al. [29]).
Research hypotheses
Before the experimental tests, we assessed the observations proposed by Horowitz et al. [25]. As a preliminary study, we tested the superiority of the “Top Down Processes” hypothesis. We compared the visual attention performance of 10 PD patients with predictable and unpredictable stimuli. As described by Horowitz et al. [25], when PD patients are informed in advance about the target characteristics they are faster than when they must detect equally salient but unpredictable targets. As a consequence, the target stimuli in our experiments were chosen accordingly to our observations in the preliminary study. In study 1 we investigated the risk of confusing selective attention with motor impairments when using classical cancellation tasks with PD patients. We administered a cancellation task designed to separate the motor component from visual attention, in order to assess its effects on the performance. Therefore, we hypothesised that traditional cancellation tasks, imposing a great motor demand to PD patients, would result in bad performance due to decision and motor delays, rather than to actual impairments in the visual attention for a predictable and salient target.
This could demonstrate that the cancellation tests currently used in clinical diagnostics are ill-suited for the assessment of selective attention and visual attention. In study 2 we investigated indirectly, the style of visual attention rescaling in PD patients by means of the Navon test [30], as suggested by Humphreys & Riddoch [31]. We aimed at exploring with clinical tests some specific features of the visual attention in PD patients, as suggested by literature. All clinical participants in the various experiments reported here were recruited from San Martino Hospital in Genoa, and both the control and experimental groups and received a detailed explanation of the procedure in accordance with the Declaration of Helsinki.
Preliminary study
In this study we wanted to confirm that PD patients perform better when they know in advance the target characteristics, since they take advantage of a “Top Down” condition that is not present when the singleton can change its features (Horowitz et al. [25]). In addition, targets have been chosen well above the discrimination threshold in comparison with distractors.
Participants
The group was formed by 10 PD patients (3 females), mean age 69 years, with normal cognitive level (MMSE>24) and ranking between 1 and 3 at the Modified Hoehn & Yahr scale [32]. We are aware that the preliminary experiment does not have a control group. However, by evaluating with this same test the simple assertion of the “Pop-Out” effect in non-pathological subjects, we only wanted to verify the effect found by [25] on subjects with Parkinson’s disease, and we have obtained confirmation of it (Table 1).
Table 1: Mean RTs and standard deviation in the bottom-up and the top-down conditions in a group of 10 participants.

Materials and Methods
The stimuli were frames as those depicted in Figure 1. They were 6x6cm squares, equivalent to 6° of visual angle when viewed at a distance of 57cm from the screen. Within the squares were displayed the target stimulus (5mm high, 3,5mm width) and 5, 15 or 30 distractors of the same size. The square is displayed after a 1000ms blank and is visible until the participant’s response. After the response, a new trial starts with another 1000ms blank screen. The three conditions of set size of distractors (5,15,30) are randomized and can be displayed according the two following conditions:
Figure 1:Example of trial in the “Bottom-Up” and the “Top-Down” condition. Participants were asked to press the key as fast as they could, according to the side of the diamond’s cut. The visual attention was based on the “Pop Out” effect, since the target was clearly standing out among the distractors. In the “Bottom-Up” condition, instructions asked to search for the diamond differing from the others, thus giving prevalence to exogenous factors. In the “Top-Down” condition, on the other hand, instructions specified the target’s features (e.g., a red diamond), endogenously setting the observers towards it. Note: the original colours were red (dark diamonds) and green (light diamonds).

a) Bottom-up condition:Two blocks, both formed by 24 randomized trials. Participants received the following instructions: You will see a diamond having a different colour from the others displayed on the screen; please press as fast as possible the ‘Q’ key on the keyboard if the diamond’s cut is on the left, and the ‘P’ key if the cut is on the right. The blocks were both formed by 24 trials with 8 trials per each of the 3 set size conditions. There were 24 targets (12 red and 12 green), 6 with the cut on the left side and 6 with the cut in the right side.
b) Top-down condition:Two blocks, both formed by 24 randomized trials. Participants received the following instructions: You will see a green diamond surrounded by red diamonds; please press as fast as possible the ‘Q’ key on the keyboard if the diamond’s cut is on the left, and the ‘P’ key if the cut is on the right. The blocks were both formed by 24 trials with 8 trials per each of the 3 set size conditions, 12 with the cut on the left side and 12 with the cut on the right side. The same instructions were provided in the following block, but the target diamond changed its colour from green to red. The total number of trials in the two blocks is 48. Before the experimental block, participants underwent a practice block, with 6 trials in the “Top-Down” condition and 6 trials in the “Bottom- Up” condition. All the conditions were counterbalanced across participants.
Result
As shown in Table 1, the RT in the bottom-up condition are higher than in the top-down condition. The data confirm part of what was found by Horowitz et al. [25]) with slightly different paradigm, the only difference is between the top-down and the bottom-up condition, with no “Display Size” effect. The RT distribution for each condition is normal, since the Kolmogorov- Smirnov test is not significant in any condition (p is comprised in a range from .878 and .988).
The experimental design was a 2 (top-down and bottom-up condition)x3 (5, 15, 30 distractors). Therefore, we performed a repeated measures ANOVA for both the bottom-up and the topdown condition and the three levels of display size. The Mauchly test for sphericity was significant for each level and we adopted the conservative Greenhouse-Geisser correction (F=21,208 (2,166); p<.0001).The partial eta-squared (=.702) provided an effect size with a power=1. Therefore, there are significantly different RTs between top-down and bottom-up conditions. The post hoc comparison (Least Significant Difference) within conditions showed no significant differences among display sizes.
Discussion
The RTs of the top-down and bottom-up conditions show a similar pattern, since they are parallel to the abscissa. This means that a pop-out effect was present for PD patients, because the RTs do not increase in relation with the display size. Connecting the data points relative to the display size with 5 and 15 distractors in the two conditions, we get two lines: For the bottom-up condition y=-0.08x+2454; and for the top-down condition: y=-0.19 x+1561. Taking into account the intercept (where there is not visual attention, since there are not distractors), we can estimate the difference between the two intercepts as 893 ms (2454 -1561). Such a considerable difference could be due to the choice time and the movement time, as suggested by Horowitz et al. [25]. PD patients in the bottom-up condition are more hesitant in their responses. One limitation of the preliminary study is the absence of a control group; however, in non-pathological subjects, the test shows the expected effects. We may argue that the RTs increase could be due to decision and motor processes activated by the absence of instructions that could allow the participants figuring out target’s visual attributes. In order to control and remove this variable, we developed further experiments where the targets were previously described to the participants.
Study 1
In this experiment we wanted to investigate whether the PD patients’ performance in cancellation tests is due to the motor component of the task or to the selective attention, as claimed by many cancellation tests with targets like numbers [27], letters [26], and figures [28].
Participants
The experimental group was composed by 15 patients in treatment for idiopathic Parkinson disease. The mean age was 69, they were 10 male and 5 females, with normal cognitive level (MMSE>24) and Hoehn & Yahr [32] modified stadiation level from 1 to 3. The control group was composed by 15 participants (8 females), mean age 67 years, and normal cognitive level (MMSE>24). In order to assess the age distribution in the two groups, we carried on an unequal variance t-test and we observed that there are no significant differences among them (t(21, 6)=-1.109; p=.280).
Materials and Methods
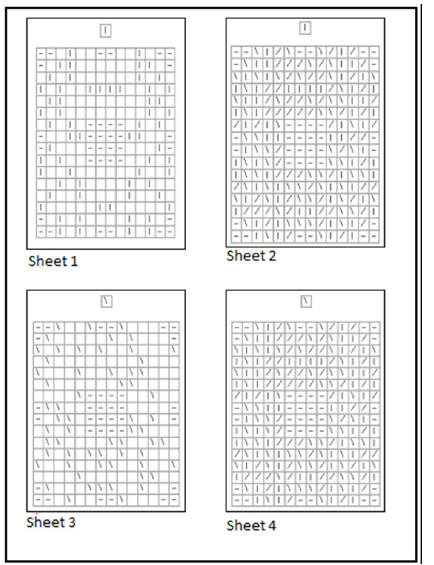
In order to investigate focused selective attention [23], we adopted a cancellation test which can discriminate motor skills from visual attention (MEA battery, Benso et al. [33]). The task is based on the cancellation of target stimuli with a red pen. The target features are displayed at the top of the sheet (top-down condition) and the participant must cancel each target as fast as possible remaining within the target’s frame (this requires just a little of motor control). There are 4 sheets, some of them are based on simple visuomotor skills without any involvement of visual attention strategies, because the targets are clearly visible and distinguishable (as can be seen in sheet 1 and 3, in (Figure 2)). The horizontal bars serve as spatial orientation facilitators as they delineate the boundaries and initially attract attention to the center. Other sheets, on the contrary, need a complex visual attention together with the motor skills to cross the target, because they are embedded among distractors (as can be seen in sheets 2 and 4 in (Figure 2)).
Figure 2:Sample sheets from the cancellation test used in our experiment. These are the most representative sheets of the test. Sheet 1 and 3 measure motor speed in a simple cancellation task, while sheets 2 and 4 measure the visual attention strategy together with the motor speed.
For each sheet, the cancellation time (t) is divided by the number of correctly crossed targets (pt). The result is the time needed to cancel and/or explore a single target. The visual attention to select the target is computed subtracting from a complex task (composed by motor skills and visual attention, like in sheets 2 and 4) the results obtained in a simple motor task (sheets 1 and 3), as shown in the formulas in Table 2.
Table 2: Sheets 2 and 4 are similar to most of the visual attention tasks. The performance in sheet 1 (simple motor task 1) has been subtracted to sheet 2 (a combination of motor and visual attention tasks 1) in order to investigate the visual attention less influenced by the motor component, concerning the first target (|). The same algorithm is repeated for the second target (\), where visual attention is computed subtracting the performance in sheet 4 (combining motor and visual attention tasks) with that in sheet 3 (simple motor task 2).

Result
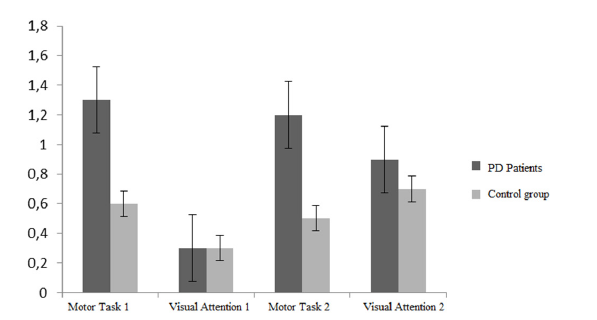
As shown in Table 3 and in Figure 3, the performance computed as time per target is significantly worse for PD patients only in the simply motor tasks. The normality of the data distribution across the four conditions for each group was confirmed by the Kolmogorov- Smirnov test (p>.05). Kurtosis and skewness are distributed within the interval (+1 and -1) as a further demonstration of distributions normality (35). The homogeneity of variance was then assessed by means of the Levene’s test. No homoscedasticity was found among groups in the experimental conditions, therefore we adopted an unequal variance t-test. The results showed a significant difference between the PD patients group and the control group concerning the task combining the visual attention and the motor control, both with the vertical stimulus (sheet 2), t(14,67)=-3,82; p=.002, and with the tilted stimulus (sheet 4), t(15,70)=-3,02; p=.008). These results are similar to those obtained with the majority of cancellation test used in clinical research, where the motor component and the visual attention strategy are enmeshed. According to these results PD patients should have selective attention deficits. However, as shown in the chart in Figure 3, when the motor component is filtered out and the visual attention task is taken into account, the two groups have similar performances: Visual attention 1, t(15,942)=-.470; p=.645; Svisual attention 2, t(18,49)=-1,22; p=.240. The consistent difference is related to the fine motor skills, as expected. The comparison of the motor skills of the two groups gave the following results: Motor skills 1, t (14,69)=- 4.170; p=.001; motor skills 2, t (14,85)=-3.671; p=.002. In order to avoid the familywise error applying Benjamini-Hochberg’s [34] correction34, adjusted significance level is p=.05; all differences remained significant. Furthermore, the ‘d’ value of the effect size showed a large according to Cohen’s guidelines [35]. These results bring further evidence to the hypothesis that PD patients underperformance in cancellation tests are mainly due to motor impairments.
Table 3: Performance for the experimental and control group. The motor task 1 and 2 performance are computed as the time for sheet completion divided by the number of targets. The performance for the visual attention task 1 and 2 is computed according to the formula previously described in table II. The only significant difference between PD patients and the control group is concerning the motor tasks, while the visual attention performance is similar in both groups. The table reports the means, standard deviations (in parentheses), effect size (d) and Benjamini-Hochberg correction.

Figure 3:Diagram comparing the performance in cancellation test of the PD patients’ group and the control group.
Discussion
A preliminary data analysis on the cancellation performance could erroneously suggest that PD patients have problems in visual attention. However, filtering out the motor component we were able to demonstrate that the differences were due to simply motor impairments typical of PD patients and that the serial visual attention task was comparable with that of the control group. The task demands in our experiment were designed in order to provide the best conditions to maintain a focused attention: visual contrast, previous description of the target’s feature, serial visual attention. We acknowledge that cancellation tasks are more sensitive to the motor impairments than the task used in the preliminary study and in Horowitz et al. [25], where the motor demand was just concerning the press or release of a key to assess choice reaction times.
The results of our study suggest that underperformance in cancellation tests could be imprudently interpreted as a sign of attention impairment, not only for Parkinson’s disease. The motor component of the response should be carefully monitored and measured. As argued by Weiskrantz R et al. [35,36] this could be a “Task Impurity Problem” since the peripheral modular intervention of input and output systems during the task will inevitably bias the measurement of attentive executive functions. However, in our study, we filtered out the effect of motor skills on the measurement of attention, because we measured it in sheets 1 and 3, and then we subtracted it to the performance in sheets 2 and 4.
Study 2
The results of study 1, and further analysis of literature, could suggest the hypothesis of a narrowed focus of attention in PD patients. First of all, in our study, the experimental and control group had a similar performance in the cancellation task requiring a focused attention by means of a stimulus by stimulus search. Secondarily, hesitations in performance based on a distributed attention has been observed in PD patients [19,20]. Furthermore, Harris et al. [24] demonstrated that PD patients might have problems with contrast sensitivity in the peripheral visual field. In order to test this hypothesis, we planned to administer a Navon test [30], as suggested by Humphreys & Riddoch [31]. The more specific measure of attentional focus (Benso et al. [37]) would be too abstract, so we prefer to investigate the style of visual attention, which can emerge in a test like Navon’s [30]. It is worth noting that the article titles Forest before trees: The precedence of global features in visual perception.
Participants
The experimental group was composed by 24 PD patients (9 females). The mean age was 73, with normal cognitive level (MMSE >24) and Hoehn & Yahr [32] modified stadiation level from 1 to 3. The control group was composed by 20 participants (8 females), mean age 71 years, and normal cognitive level (MMSE>24). In order to assess the age distribution in the two groups, we carried on an unequal variance t-test and we observed that there are no significant differences among them (t(42) =.868; p=.390).
Materials and Methods
In order to investigate the focusing style, we adopted the Navon test, as suggested by Humphreys & Riddoch [31]. Participants are required to press as fast as possible a key on the keyboard, according to the instructions. The task is divided in 4 blocks, 2 for each condition (local and global), with 20 trials each. Half of the trials is congruent, with the large letter composed by the same small letters. The other half of the trials is incongruent, with the large letter composed by different small letters, as shown in Figure 4. At the beginning of each block, participants are requested to pay attention either to the global letters (large H and F), or to the local letters (small Hs and Fs).
Figure 4:The Navon test. The task is composed by two conditions (global and local) with two levels (congruent and incongruent). Participants are required to pay attention and discriminate either the large letters (global task) or the small letters (local task).

According to the evidences found in literature, healthy participants are faster in the congruent situation than in the incongruent. However, the trend is not symmetrical among the “Global” and “Local” conditions, since participants are faster and more accurate when they are asked to respond to global letters rather than local, most of all when in the incongruent conditions. This is based on a phenomenon named “Precedence of Global” [30]. Several kinds of impairments have been investigated in patients that showed a “Precedence of Local” phenomenon. This led to the hypothesis of a narrowed attentional focus, since subjects were not disturbed by the incongruent global letter that had not been properly processed [31].
Result
The response times are therefore weighted for the number of errors. Table 4 and Figure 5 show the results computed according to this formula. The Kolmogorov-Smirnov test demonstrated the normal distribution of the variables (p>.05), also confirmed by the kurtosis and skewness ranging between -1 and 1. The only exception is concerning the distribution of RT for the incongruent local condition in PD patients (p=.04; skewness: 1,78; kurtosis: 2,64). Notwithstanding this small anomaly, we decided to adopt parametrical tests however, very robust. The comparison of RT times showed, as expected, that PD patients were slower than the control group. The most interesting aspect, however, is not the comparison between groups, but the investigation of RT within groups, as suggested by Humphreys and Riddoch, 198731. The effectiveness of the control group showed the Navon effect, as shown in Figure 4: Participants were faster in the congruent rather incongruent conditions, most of all the RT of the global condition were significantly faster than those of the local one. A repeated measures t-test comparing local and global conditions showed a significant difference, confirming the “Precedence of The Global”: t(39)=-3,22, p=.003. The effect size ‘d’=0.50 is medium according to Cohen’s guidelines [35].
Table 4:Congruent and incongruent’s local and global RTs, mean and standard deviation in parenthesis of the two groups.
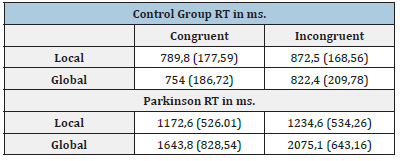
Figure 5:The “Navon Effect” in the control (A) and experimental group (B). As showed in Figure 5A, the control group have the typical trend of precedence of the global. In Figure 5B, the PD patients revert the trend, since they have an attentional focus oriented to the local dimension (with faster reaction times) rather than the global one.

Once demonstrated the Navon effect in the control group, we investigated the trend of RT in PD patients following the same statistical procedure. The experimental group showed faster RT in the congruent conditions, however, the Navon effect was reversed in comparison with the control group and a “Precedence of the Local” was observed. PD patients had faster RT in the local incongruent condition than in the global incongruent condition (as shown in Figure 5B). The repeated measures t-test showed a significant difference between the global and local conditions: t (47)=6,33; p<.0001. The effect size ‘d’=0.90 showed a large effect according to Cohen’s guidelines [38].
Discussion
As discussed in literature, PD patients have a peculiar and specific kind of visual attention. This could be partially explained by the focusing style, as shown by the inversion of the Navon effect, where local conditions have faster RTs that the global ones. PD patients break the “Precedence of the Global” rule and could tend to keep a narrowed attentional focus. This is also demonstrated by the faster RTs in the local conditions (both congruent and incongruent) rather than in the global conditions. This effect could be explained by a very low interference of large pictures. The results obtained by Harris et al. [4], might demonstrate how PD patients have a lower contrast sensitivity at the periphery of the visual field, are therefore still hypothesis. Finally, we could hypothesize that, since these patients have problems in filtering stimuli entering the working memory [39], they could keep the peripheral filter implicitly and selectively focused in order to avoid the interference of surrounding distractors [18]. Further research is needed to investigate this last hypothesis.
Conclusion
The studies described in the present paper provided further evidence to phenomena described in literature concerning the attentive style of PD patients. In addition, we observed new and interesting phenomena that could be very useful for a better understanding of the disease. In the preliminary study we replicated in part the results obtained by Horowitz et al. [25], assessing the PD patients’ difficulty in responding to “Pop-Out” singletons displayed without any previous description of their features. The analysis of the intercept of RTs demonstrated higher RTs in the bottom-up than in the top-down condition, due to the motor hesitations, as similarly described by Horowitz et al. [25].
In the first study we investigated the validity of the cancellation test. Our results showed a significant difference in the general performance at the cancellation test, in accordance with the vast majority of cancellation tests used in the clinical research. However, filtering out the motor component form the serial visual attention component of the performance, we observed that PD patients had performance as good as the one of control group when the visual attention was stimulus by stimulus. On the other hand, we observed a statistically significant difference between the control and the experimental group just in the motor component, predictably less efficient in PD patients. These results led us to reconsider the validity of cancellation tasks in clinical research, where the motor and the visual attention component are merged in a task based on the serial search of targets embedded among distractors. These tasks could misinterpret attentional deficits by means of the delay due to the motor component, which is notoriously impaired in PD patients. When the role of the motor component is relevant, like in study 1 (crossing a target) with respect to the preliminary study (pressing a key), PD patients could be disadvantaged. In other words, cancellation tests could be biased by the confusion of motor and attentive components (the “Task Impurity Problem” [37-39]). Some clinicians and researchers could be convinced of measuring a central processing (the selective attention), while actually measuring a performance biased by a confounding peripheral variable (the motor control), necessary to execute the task.
The study 2 was developed after a critical review of literature in light of what we observed with PD patients [22,24,25]. Following these evidences, we hypothesised that PD patients could have a narrowed attentional focus also when they should distribute it over the visual field. In order to investigate this hypothesis, we adopted the Navon test [30], as suggested by Humphreys & Riddoch [31]. As expected, PD patients inverted the Navon effect: They were faster in the recognition of small letters that the larger ones. Therefore, they were more efficient in the local condition than in the global condition, reverting the normal phenomenon of the “Precedence of the Global”. This leads us to argue that PD patients could adopt narrowed focusing styles, since they were not disturbed by the large incongruent letters, as observed in the control group.
Limitations and Future Proposals
We are aware that it would have been more elegant to save the data from the control groups for the ‘Pop-Out’ effect experiment following the works of Horowitz et al. [25]. However, it was a deliberately defined ‘Preliminary Experiment’ as a control step. In a future study, given the data and hypotheses that have emerged, it could be interesting to try to correlate structured observations on gait and certain fundamental movements detectable in subjects with Parkinson’s disease with aspects of visual attention related to the size of focus. Several aspects deserve further investigation. For instance, further research is needed on the size of attentional focus and, moreover, investigating its relationship with the neurofunctional impairments. McNab & Klingberg [40]) demonstrated the relevance of dorsolateral prefrontal cortex and basal ganglia for filtering irrelevant information in working memory. Awh & Vogel [41] reinforced this role of basal ganglia describing them as the “Bouncers” in our brain. Lee et al. [42] confirmed the difficulty of PD patients in filtering distractors and the role of basal ganglia in the control of information incoming the working memory. Framing these evidences in the Lavie’s theory [18], we could argue that the (peripheral) perceptual filter could afford a kind of protective compensation, narrowing the attentional focusing and, therefore, containing the load of irrelevant stimuli present in the visual field. This could help the central filter, operating at the working memory level, which has been weakened by the basal ganglia impairments observed in PD patients [41,43].
For some of these conjectures, we propose new experiments that we are also evaluating through careful clinical observations. We are monitoring everything by conducting test-based investigations during intervention phases where we utilize the Integrated Cognitive Training (ICT) of the “Benso Method®” [44,45]. This method has demonstrated its effectiveness through PET scans with amnestic MCI elderly individuals (Ciarmiello et al. [46]). “Benso Method®” involves in-depth clinical investigations even during treatment. It is utilized in various settings with different types of interventions and calibrations for neurodevelopmental disorders, adult brain trauma, degenerative issues in the elderly, academic and amateur performance enhancements. All of this provides us with diverse quantitative and qualitative information (as in the case of this paper on visual attention) that we plan to frame with controlled experiments to promote further developments in intervention applications.
This specific focusing style on PD patients could therefore play a relevant role also in other behaviours like the motor hesitations and the freezing. The influence of visual perception on motor skills has been hypothesised by other studies as well [47], where it was observed the importance of the visual feedback for the control of motor hesitations and postural attitude. In addition, this evidence of the relevant role of the visual attention style on motor skills should be taken into account both regarding the study of possible predictive indices and in the development of rehabilitation and compensation techniques, aimed at reducing the attentional impairments of PD patients and at providing them with a better quality of life.
Acknowledgment
Patrizia Valle, for the contribution provided in data collection.
References
- Wollner IB, Yahr MD (1978) Measurements of visual evoked potentials in Parkinson’s disease. Brain 101(4): 661-671.
- Marx M, Wollner IB, Bobak P, Harnois C, Mylin L, et al. (1986) Temporal frequency-dependent VEP changes in Parkinson’s disease. Vision Research 26(1): 185-193.
- Ghilardi MF, Marx MS, Wollner IB, Camras CB, Gloveret AA (1989) The effect of intraocular 6-hydroxydopamine on retinal processing of primates. Ann Neurol 25(4): 357-364.
- Ikeda H, Head GM, Ellis CJ (1994) Electrophysiological signs of retinal dopamine deficiency in recently diagnosed Parkinson’s disease and a follow up study. Vision Research 34(19): 2629-2638.
- Elst LTV, Greenlee MW, Foley JM, Lücking CH (1997) Contrast detection, discrimination and adaptation in patients with Parkinson’s disease and multiple system atrophy. Brain 120(12): 2219-2228.
- Masson G, Mestre D, Blin O (1993) Dopaminergic modulation of visual sensitivity in man. Fundamental & Clinical Pharmacology 7(8): 449-463.
- Wollner IB, Onofrj M (1987) Visual system in Parkinson’s Disease. Advances in Neurology, In: Yarh MD, Bergmann KJ (Eds.), 45: 323-327.
- Papadopoulos GC, Parnavelas JG (1990) Distribution and synaptic organization of dopaminergic axons in the lateral geniculate nucleus of the rat. Journal of Comparative Neurology 294(3): 356-361.
- Reader TA, Quesney LF (1986) Dopamine in the visual cortex of the cat. Experientia 42(11-12): 1242-1244.
- Mestre D, Blin Q, Serratrice G, Pailhous J (1990) Human Spatio-temporal contrast sensitivity differs in normal aging and Parkinson’s disease. Neurology 40(11): 1022-1023.
- Cherry EC (1953) Some experiments on the recognition of speech, with one and with two ears. The Journal of the Acoustical Society of America 25: 975-979.
- Broadbent DE (1958) Perception and communication. Elmsford, NY, US: Pergamon Press, UK.
- Deutsch JA, Deutsch D (1963) Attention: Some theoretical considerations. Psychological Review 70(1): 80-90.
- Duncan J (1980) The locus of interference in the perception of simultaneous stimuli. Psychological Review 87(3): 272-300.
- Duncan J (1981) Directing attention in the visual field. Perception & Psychophysics 30(1): 90-93.
- Tipper SP, Driver J (1988) Negative priming between pictures and words in a selective attention task: Evidence for semantic processing of ignored stimuli. Memory & Cognition 16(1): 64-70.
- Lavie N, Tsall Y (1994) Perceptual load as major determinant of the locus of selection in visual attention. Perception & Psychophysics 56: 183-197.
- Lavie N (2000) Selective attention and cognitive control: Dissociating attentional functions through differents types of load, In: Monsell S, Driver J (Eds.), Attention and performance XVIII, Cambridge, MA, The MIT Press, USA, pp. 175-194.
- Troscianko T, Calvert J (1993) Impaired parallel visual search mechansims in Parkinson’s disease: Implications for the role of dopamine in visual attention. Clinical Visual Science 8: 281-287.
- Weinstein A, Troscianko T, Calvert J (1997) Impaired visual search mechanisms in Parkinson’s disease (PD): A psychophysical and event-related potentials study. Journal of Psychophysiology 11: 33-47.
- Berry MD (1999) R-2HMP, an orally active agents combining independent anti-apoptotic and MAO-B inhibitory activities. CNS Drug Review 5: 105-124.
- Lieb K, Brucker S, Bach M, Els T, Lücking CH, et al. (1999) Impairment in preattentive visual processing in patients with Parkinson’s disease. Brain 122(2): 303-313.
- Bravo MJ, Nakayama K (1992) The role of attention in different visual-search tasks. Perception & Psychophysics 51(5): 465-472.
- Harris JP, Calver JR, Phillison OT (1992) Processing of spatial contrast in peripheral vision in Parkinson’s disease. Brain 115(5): 1447-1457.
- Horowitz TS, Choi WY, Horvitz JC, Côté JL, Mangels JA (2006) Visual search deficits in Parkinson’s disease are attenuated by bottom-up target salience and top-down information. Neuropsychologia 44(10): 1962-1977.
- Diller L, Weinberg J (1977) Hemi-inattention in rehabilitation: The evolution of a rational remediation program. In: Wenstein EA, Friedland RP (Eds.), Hemi-inattention and hemisphere specialization. Adv Neurol 18: 63-82.
- Spinnler M, Tognoni G ( 1987) Standardized a taratura italiana D test neuropsychologica. The Italian Journal of Neurological Science 8: 6.
- Gauthier L, Dahaut F, Joanette Y (1989) The Bells test: A quantitative and qualitative test for visual neglect, Neuropsychology. International Journal of Clinical Neuropsychology 11: 49-54.
- Lollo VD, Kawahara JI, Zuvic SM, Visser TAW (2001) The preattentive emperor has no clothes: A dynamic redressing. Journal of Experimental Psychology General 130(3): 479-492.
- Navon D (1977) Forest before trees: The precedence of global features in visual perception. Cognitive Psychology 9(3): 353-383.
- Humphreys GW, Riddoch MJ (1987) To see but not to see: A case study of visual agnosia. London Lawrence Erlbaum, UK.
- Hoehn M, Yahr M (1967) Parkinsonism: Onset, progression and mortality. Neurology 17(5): 427-42.
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B 57(1): 289-300.
- Weiskrantz L (1992) Unconscious vision. The Sciences 32(5): 22-28.
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, et al. (2000) The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: A latent variable analysis. Cognitive Psychology 41(1): 49-100.
- Benso F, Turatto M, Mascetti GG, Umiltà C (1998) The time course of attentional focussing. European Journal of Cognitive Psychology 10(4): 373-388.
- Cohen J (1988) Statistical power analysis for the behavoral sciences, 2nd (edn.) New York: Lawrence Eribaum Associates, USA.
- Lee EY, Cowan N, Vogel EK, Rolan T, InclanVF, et al. (2010) Visual working memory deficits in patients with Parkinson's disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain 133(9): 2677-2689.
- McNab F, Klingberg T (2007) Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience pp.1-5.
- Awh E, Vogel E (2008) The bouncer in the brain. Nat Neurosci 11: 5-6.
- Muthén B, Kaplan D (1992) A comparison of some methodologies for the factor analysis of non-normal Likert variables: A note on the size of the model. British Journal of Mathematical and Statistical Psychology 45(1): 19-30.
- Benso F, Santoro GM, Ardu E (2019) MEA: Measures for executive attention. Hogrefe, Italy.
- Rabbitt P (1997) Introduction: Methodologies and models in the study of executive function. In: Rabbitt P (Ed.), Methodology of frontal and executive function, Hove, East Sussex: Psychology Press, UK, pp. 1-38.
- Benso F, Moretti S, Bellazzini V, Benso E, Ardu E, et al. (2021) Principles of integrated cognitive training for executive attention: Application to an instrumental skill. Frontiers in Psychology 12: 2223.
- Benso F, Benso E (2023) The neuroscientific principles of cognitive training. Applications of the Benso Method® in the clinic, in Educational, Neuromotor, Sports and Artistic Fields. Hogrefe, Italy.
- Ciarmiello A, Gaeta MC, Benso F, Sette MD (2015) FDG-PET in the evaluation of brain metabolic changes induced by cognitive stimulation in MCI subjects. Current Radiopharmaceuticals 8(1): 69-75.
- Jacono M, Baratto L, Simonini M (2005) Criteria for the analysis of a sample of normality in posturography.
© 2023 Eleonora Ardu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






