- Submissions

Full Text
Experiments in Rhinology & Otolaryngology
Endoscopic Transorbital Cauterization of the Anterior Ethmoidal Artery
Carlos Santiago R*, Lourdes Principe and Ana Laura Cajelli
Department of Otolaryngology, Hospital Italiano de Buenos Aires, Argentina
*Corresponding author: Carlos Santiago Ruggeri MD, Department of Otolaryngology, Chief of Rhinosinusology division, Hospital Italiano de Buenos Aires - Gascón 450, CP1181, CABA, Argentina
Submission: July 10, 2018;Published: September 26, 2018

ISSN 2637-7780 Volume2 Issue4
Abstract
Objective: Determine the effectiveness of Anterior Ethmoidal Artery endoscopic transorbital cauterization for recurrent posterior epistaxis treatment.
Study Design: Descriptive prospective.
Materials and methods: All the patients treated for recurrent posterior epistaxis by endoscopic transorbital cauterization in the Rhinosinusology division of the Otolaryngology department of the Italian Hospital of Buenos Aires between June 2007 and May 2017 were included.
Results: Forty-Five patients underwent an endoscopic surgical procedure for posterior epistaxis between June 2007 and May 2017. 23 of them were men and 22 women, the average age was 65 years. In 33 cases, only the SA was cauterized. In 12 cases, both the SA and the AEA as it traveled through the skull base. Three of the 12 patients in whom SA+AEA was cauterized had recurrent epistaxis. In two of them the AEA was cauterized with endoscopic transorbital technique. Both were women of 64 and 66 years old. The time of hospitalization after the surgery was 48 hours and there were no complications. They were controlled without referring any new bleeding.
Conclusion: The effectiveness of cauterization of the anterior ethmoidal artery performed by endoscopic transorbital approach due to recurrent posterior epistaxis was 100%. We consider that due to the anatomical variability of the artery and the greater possibility of complications with other surgical techniques, the endoscopic transorbital approach is the best option to resolve the posterior epistaxis when the origin is the anterior ethmoidal artery. Due to the few studies published and small series of patients, the role of this surgical technique depends on the results of a greater number of patients treated through this approach.
Keywords: Anterior; Ethmoidal; Artery; Endoscopic; Surgery
Abbreviations: AEA: Anterior Etmoidal Artery; SA: Sphenopalatine Artery; AEC: Anterior Ethmoidal Canal
Introduction
The posterior epistaxis is a frequent disease that is, in most cases, idiopathic. The hemorrhage in this case is most often caused by the sphenopalatine artery (SA). It is no so common that the anterior etmoidal artery (AEA) causes the bleeding. Most surgical treatment protocols for posterior epistaxis do not include the cauterization of the anterior ethmoidal artery in the first stage, except when an antero-superior bleeding point is evident in the nasal cavity, in front of the middle turbinate. Moreover, because of the low incidence of AEA bleeding, there is less experience in surgical techniques, cauterization and vascular clips placing. Another important variable is the course of the artery, which may be covered with bone or can be dehiscent (located inside the cribiform plate or below it). The aim of this study is to determine the effectiveness of AEA endoscopic transorbital cauterization for recurrent posterior epistaxis treatment.
Materials and Methods
We performed a descriptive prospective study. All the patients who were treated for recurrent posterior epistaxis by endoscopic transorbital cauterization in the rhinosinusology division of the Otolaryngology department of the Italian Hospital of Buenos Aires between June 2007 and May 2017 were included. We excluded patients who were treated for posterior epistaxis by ligation or cauterization of the anterior ethmoidal artery using other techniques, as external or endonasal approach with cauterization at the anterior skull base above the ethmoidal Bulla. The data collected was recorded in an Excel 2010 table.
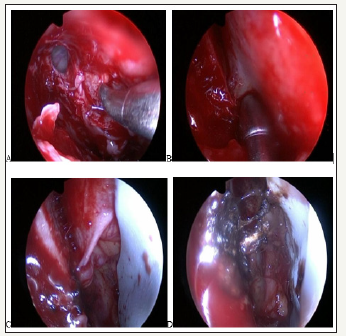
The surgeries were performed under general anesthesia. A 30°endoscope and conventional instruments for rhinosinusal endoscopic surgery were used. A maxillary antrostomy was followed by an anterior ethmoidectomy and localization of the frontal drainage. Once the lamina papyracea was identified, a minimal medial orbitotomy was performed, resecting it. The orbitotomy had as limits: the frontal ostium (anterior), the skull base (superior), the roof of the ethmoidal bulla (inferior) and 1 cm from the frontal ostium (posterior). The periorbit was then dissected, trying not to produce an intraorbital fat hernia, and the AEA was in its emergence from the eye through the periorbita, on its way to the nasal cavity. An eye shield was inserted through the nostril to avoid possible damage to the eye by heat, and the artery was cauterized with monopolar or bipolar diathermy (Figure 1). The cauterization of the SA was re-evaluated. Hemostatic matrix with thrombin (Surgifló) was then placed in the nasal cavity to achieve hemostasis.
Figure 1:Endoscopic transorbital cauterization of the AEA.
A. Orbitotomy
B. y
C. AEA inside the orbital cavity
D. AEA Cauterization
Results
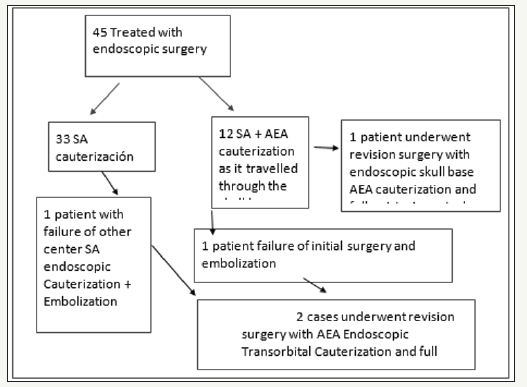
Forty-Five patients underwent an endoscopic surgical procedure for posterior epistaxis between June 2007 and May 2017. 23 of them were men and 22 women, the average age was 65 years. In 33 cases, only the SA was cauterized. In 12 cases, both the SA and the AEA as it traveled through the skull base. Three of the 12 patients in whom SA + AEA was cauterized had recurrent epistaxis. In two of them, the anterior ethmoidal artery was cauterized again (in one also with the posterior ethmoidal artery) after the failure of the surgery (cauterization of the SA+AEA) and embolization. In another patient AEA was cauterized after the failure of cauterization of the SA and embolization performed in another center. One of these 3 patients achieved full epistaxis control after endoscopic cauterization of AEA, but we excluded him because the artery was cauterized via the endonasal route at the skull base. The two that were included were evaluated by clinical examination, hematological studies, and computed tomography. In two patients, the AEA was cauterized with endoscopic transorbital technique. Both were women of 64 and 66 years old. The time of hospitalization after the surgery was 48 hours and there were no complications. They were controlled for 4 months without referring any new bleeding.
Discussion
The posterior epistaxis is a frequent urgency in otorhinolaryngological consult. There are different treatments, indeed is well know that surgical treatment has better results (90% success) than antero-posterior nasal packing (62%), fewer complications, and the length of hospitalisation is shorter (average 24 hours after surgery, versus 4 days after packing). Embolization by digital angiography, in comparison with surgery has an inferior result (75%) therefore can be the first choice in patients who have a high risk to undergo general anesthesia [1]. Although is not a possible option for bleeding of AEA because of the risk blindness. Bleeding from the SA, which arises from the maxillary artery and is the terminal branch of the external carotid system is the is most frequent cause of posterior epistaxis. On the other hand, epistaxis that originates from the ethmoidal arteries is rare. These arteries arise from the ophthalmic artery, which is the terminal branch of the internal carotid artery, so as it was said they cannot be embolized in cases of nosebleeds. The anterior ethmoidal artery leaves the eye through the peri-orbit and the lamina papirácea of the ethmoid bone, pass through the anterior skull base in a diagonal direction, going from postero-lateral to antero-medial in the anterior ethmoidal canal (AEC).
It is located between the second and third lamella of the ethmoid bone, above the ethmoidal bulla and behind the frontal ostium. It can also be located behind a supraorbital cell, which represents an extension of the suprabullar space. If the ethmoidal bulla is attached to the skull base, and there are no recesses, then the AEC is in the junction of the bulla and the skull base. Upon leaving the AEC, the AEA extends over the cribriform plate and gives the anterior meningeal branch. Then it enters the nasal cavity through the anterior ethmoidal foramen of the lamina cribosa and ends up branching. The average diameter of the AEA, on the right side is 0.92+/-0.2mm and on the left side is 0.88+-0.15mm. The average length at the intranasal level is 5.82+-1.41mm [2]. Along with the Posterior ethmoidal Artery, it supplies the external and internal nasal wall in its upper sector. For patients presenting with posterior epistaxis, when the bleeding site is not identified, the most frequent surgical treatment consists of cauterizing the SA by endonasal approach with monopolar or bipolar diathermy. This is based on the greater frequency of hemorrhages originated from the SA, the greater territory of nasal irrigation and the greater caliber of the vessel compared with the anterior ethmoidal artery. In our group, only in cases refractory to the cauterization of the SA or when the bleeding site was identified in front of the middle turbinate, the treatment of the anterior ethmoidal artery was performed. There are multiple surgical techniques to perform AEA cauterization: the external approach is performed through an incision in the inner corner of the eye (Lynch), the periosteum is dissected, and the artery is located at the level of the frontoethmoidal suture, 2cm behind the orbital rim. This technique can be endoscopically assisted [3]. The transcaruncular technique is another option for an external approach [4]. Endoscopic endonasal surgery is the preferred one because of its lower morbidity (it avoids scars, lesions of the lacrimal route, and swelling of the eye), and offers a better view (magnification and angle).
The relationship of the AEA with the anterior cranial base is variable. A literature review, which included 542 AEAs, reported that 66.6% was founded at the skull base and 34.4% below it. They also found absence of supernumerary branches [2]. In another study they reported that 83.3% of AEAs were attached to the ethmoid roof, 4.1% were located at 2.5 to 5mm and 12.5% more than 5mm from the skull base [5]. The importance of these findings is that if the AEA courses below the base of the skull it would be more vulnerable to injury during endoscopic surgery, and on the other hand it would be feasible to cauterize or clip the artery successfully and with less possibility of complications (Figures 2&3).
Figure 2:AEA and the variable relation with skull base.
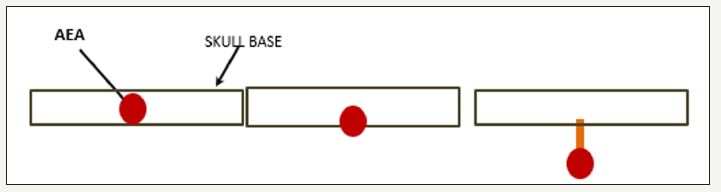
Figure 3:A. AEA below the skull base.
B. AEA inside a bony path in the skull base.
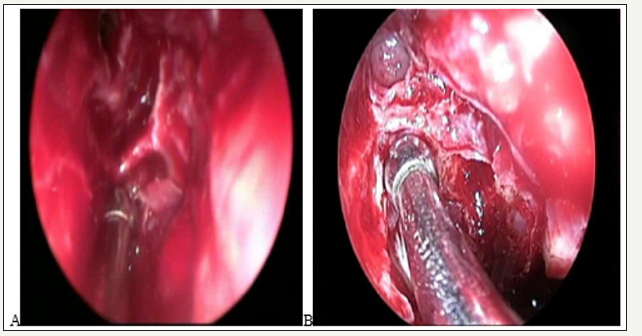
Flore ani and Wormald studied 44 nasal cavities of 22 cadaveric heads with computed tomography and 4 mm endoscopes of 0° and 30° and tried to place clips in the anterior ethmoidal artery. The artery canal passed through a bone mesentery in some section of its path in 36% of cases, and in 7 of 44 cases it was partially dehiscent (16%). Most of the ethmoidal arteries could not be clipated (66%), of the 36% that passed through a bone mesentery, only clips could be placed in 20%. They also found an important correlation between the increase in the height of the lateral lamella and the incidence of an anterior ethmoidal canal located in a mesentery [6]. This can be evaluated by a preoperative tomography.
There are some studies that describe how the dura mater may extend into the bone of the mesentery bone in the anterior ethmoidal canal. This would cause high risk of cerebrospinal fluid leak if it is attempted to cauterize or clip the AEA by direct transnasal route [6,7]. The endoscopic transorbital approach allows to locate the AEA in a region where it is devoid of a bony cover and far from the skull base, that is why we believe that cauterization could be more successful. When the posterior epistaxis is refractory to surgical treatment, the cauterization of the SA is usually reviewed (Figure 4). Recurrences of bleeding may occur since supernumerary branches, that may exit through the sphenopalatine foramen or through an accessory hole, remained without cauterization [8]. It is difficult in those cases to attribute success to the cauterization of one artery or another. In our series, in the review of the previous cauterization of the SA, we neither detected defects in the previously performed technique nor observed supernumerary branches of the SA. Likewise, the 2 patients we treated had a previous embolization of the SA. There are few studies that report results with endoscopic transorbital cauterization of the AEA, and the series are small. Pletcher and Metson reported 3 patients treated with this technique with resolution of epistaxis [9-11].
Figure 4:
Conclusion
The effectiveness of cauterization of the anterior ethmoidal artery performed by endoscopic transorbital approach due to recurrent posterior epistaxis was 100%. We consider that due to the anatomical variability of the artery and the greater possibility of complications with other surgical techniques, the endoscopic transorbital approach is the best option to resolve the posterior epistaxis when the origin is the anterior ethmoidal artery. Due to the few studies published and small series of patients, the determination of the role of this surgical technique depends on the results of a greater number of patients treated through this approach.
References
- Klotz D, Winkle M, Richmon J, Hengerer RB (2002) A surgical management of posterior epistaxis: a changing paradigm. Laryngoscope 112(9): 1577-1582.
- Lisbona Alquezar MP, Fernandez LR, Lorente MA, Pérez DelgadoL, Silvia HT, et al. (2010) La arteria etmoidal anterior en el laberinto etmoidal: Revisión bibliográfica sobre variantes anatómicas y referencias para la cirugía endoscópica. Acta Otorrinolaringológica Española 61(3): 201- 208.
- Douglas SA, Gupta D (2003) Endoscopic assisted external approachh anterior ethmoidal artery ligation for the management of epistaxis. J Laryngol Otol 117(2): 132-133.
- Morera E, ArtigasC, Trobat F, Ferrén L,Tomás M (2011) Transcaruncular electrocoagulation of anterior ethmoidal artery for the treatment of severe epistaxis. Laryngoscope 121(6): 446-450.
- Cunha Araujo FB, Weber R, Pinheiro Neto CD, Miranda Lessa M, Voegels RL, et al. (2006) Anatomia endoscópica da artéria etmoidal anterior: Estudo de dissecção em cadáveres. Revista Brasileira de Otorrinolaringología 72(3): 303-308.
- Floreani SR, Nair SB, Switajewski MC, Wormal PJ (2006) Endoscopic anterior ethmoidal artery ligation: a cadaver study. Laryngoscope 116(7): 1263-1267.
- Kainz J, Stammberger H (1988) The roof of the anterior ethmoid: A locus minoris resistentiae in the skull base. Laryngologie Rhinologie Otologie 67(4): 142-149.
- Simmen DB, Raghavan U, Briner HR, Manestar M, Groscurth P, et al. (2006) The anatomy of the sphenopalatine artery for the endoscopic sinus surgeon. Am J Rhinol 20(5): 502-505.
- Pletcher SD, Metson R (2007) Endoscopic ligation of the anterior ethmoid artery. Laryngoscope 117(2): 378-381.
- Pletcher SD, Metson R (2008) Endoscopic transorbital ligation of the anterior ethmoid artery. Operative Techniques in Otolaryngoly 19(3): 199-201.
- Berens AM, Davis GE, Moe KS (2016) Transorbital endoscopic identification of supernumerary ethmoid arteries. Allergy Rhinol (Providence) 7(3): 144-146.
© 2018 Carlos Santiago R. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






