- Submissions

Full Text
COJ Nursing & Healthcare
Ascorbate Deficiency among Childrens of African Descent with Protein Energy Malnutrition in Sokoto, North Western Nigeria
Erhabor O1*, Abdullahi S1, Jiya NMA2, Van Dyke K3 and Erhabor T4
1Department of Haematology, Usmanu Danfodiyo University Sokoto, Nigeria
2Department of Paediatrics, Usmanu Danfodiyo University Sokoto, Nigeria
3School of Medicine, Department of Biochemistry West Virginia University, USA
4Medical Laboratory Science Council of Nigeria
*Corresponding author: Osaro Erhabor, Department of Haematology, Usmanu Danfodiyo University Sokoto, Nigeria, Tel: +234-813-962-5990; Email: n_osaro@yahoo.com
Submission: February 26, 2018;Published: May 29, 2018

ISSN: 2577-2007 Volume3 Issue2
Abstract
Protein energy malnutrition is the most widespread nutritional deficiency disorder of mankind and continues to be a major public health burden particularly in developing countries. The aim of this case-control study was to determine the changes in the ascorbic acid levels among children with PEM in Sokoto, North Western Nigeria. The study included a total of 90 children (47 subjects with Protein Energy Malnutrition and 43 apparently healthy controls) aged 6 months-5 years, admitted to the Paediatric units of Usmanu Danfodiyo University Teaching Hospital and Specialist Hospital, Sokoto. Ascorbic acid levels were assayed by a standard chemical method. Nutritional status was determined using the Welcome Trust Classification. Data were analyzed using SPSS 22.0 statistical package. A p-value ≤ 0.05 was considered significant in all statistical comparisons. The mean value of ascorbic acid was significantly lower among subjects (0.82±0.04mg/dl) compared to controls (1.06±0.02 mg/dl) (p=0.0001). Underweight subjects had lower ascorbic acid levels when compared with other types of protein energy malnutrition (p=0.0001). Protein energy malnutrition was more prevalent among children from low socioeconomic class whose mothers have no formal education. Marasmus was the most common type of protein energy malnutrition. Finding from this study seems a justification to monitor the ascorbic acid levels in children with PEM and to possibly offer ascorbic acid supplementation for those that are deficient. There is need for infant feeding practice to be strengthened by promoting exclusive breast feeding. There is also the need for increased enrollment of women in schools, enlightenment on nutritional education and empowerment of women to improve their socioeconomic status.
Keywords: Ascorbate deficiency; Children; African descent; Protein energy malnutrition; Sokoto; Nigeria
Introduction
Nutrition may be defined as the sum of the process by which a living organism receives nutrient materials from the environment and uses them to promote its own vital activities [1]. Malnutrition continues to be a major health burden in developing countries. It is globally the most important risk factor for illness and accounts for half of children death worldwide [2]. PEM is responsible, directly or indirectly, for 54% of the 10.8 million deaths per year in children under five years of age. It also contributes to every second death associated with infectious diseases among childrens under five years of age in developing countries [3]. It was estimated that 41% of the under five children are undernourished in Nigeria with majority seen in the North Western part of the country [4].
The WHO defined malnutrition as the cellular imbalance between the supply of nutrients and energy and the body’s demand for them to ensure optimum growth maintenance and specific function [5]. Malnutrition is one of the major public health challenges in developing countries. Usually it is referred to as a “silent emergency” as it has devastating effects on children, society at large and the future of mankind. The net loss of body protein particularly skeletal muscle protein is a major factor responsible for Protein Energy Malnutrition [6,7]. Malnutrition is currently the leading cause of global burden of disease [8] and has been identified as the underlying factor in about 50% of deaths in children’s under 5 years of age in developing countries [9]. The condition may result from lack of food or from infections that cause loss of appetite while increasing the body’s nutrient requirements and losses. It is estimated that in developing countries, more than one-quarter of all children younger than 5 years of age are malnourished [10]. Malnutrition is commonly referred to as “the silent emergency and it is said to be an accomplice in at least half of the 10.9 million child deaths each year in developing countries. Of all the various forms of malnutrition, protein energy malnutrition (PEM) is considered the most lethal and infants are its targeted victim [11].
Protein Energy Malnutrition (PEM) is defined as a spectrum of diseases arising as a result of an absolute or relative deficiency of calories and/or protein in the diet [12,13]. PEM previously referred to as protein-calorie malnutrition (PCM) describes the severe forms of malnutrition seen in childhood (kwashiorkor, marasmickwashiorkor, marasmus and underweight) and the nutritionally determined growth retardation that precedes these clinical syndromes [14,15]. The two severe forms of PEM are kwashiorkor and marasmus. Kwashiorkor is due to insufficiency of good quality proteins to meet the demand of growth and tissue repairs, while marasmus is due to chronic starvation and deficiency of total calories, including proteins [16]. PEM is a major public health problem particularly in developing countries of the world and often arises during protein and/or energy deficit due to nutritional inadequacy, infections, poor socio-economic and environmental conditions [17-19].
UNICEF estimated in 2005 that malnutrition was associated with approximately 50% of child deaths worldwide. It is associated with 49% of the 10 million deaths occurring in children in the developing world and 52% of all under five deaths in Nigeria [20] with 24% and 16% of the total under-5 Nigerian population estimated to have suffered from mild-moderate and severe malnutrition respectively from 1973 to 1983.
In Nigeria, the prevalence of malnutrition among children under 5 years of age is significantly higher than in most other developing countries [21]. Demographic Health Survey conducted in 1990 by the Federal Office of Statistics [22] estimated the prevalence of wasting at 9%, stunting at 43% and underweight at 36 % among preschool children. The Nigerian demographic health survey (NDHS) conducted in 2013 [23] shows that the nutritional situation in the country was 18% wasting, 29% underweight, 4% overweight and 37% stunting. Malnutrition has serious repercussions for human development and National Productivity. It compromises physical and mental development weakens immune response and increased susceptibility to infection [24]. PEM is a complex situation that has led to a variety of methods of classification. This includes the WHO, Waterlow, Gomez, Welcome Trust/International and Modified Welcome classification. This study was based on the Welcome Trust Classification of PEM [25].
Vitamin C serves as an antioxidant to protect intracellular and extra cellular component from free radical damage. It mops up free radicals and forms the less reactive ascorbyl radical. The ascorbyl radical can then be either reduced to ascorbic acid or oxidised to dehydroascorbic acid [26]. Free radicals are highly unstable molecules that interact quickly and aggressively with the molecules in the body causing damage. The rise in free radicals, associated with antioxidant deficiency is said to result in tissue damage. The pathogenesis of oedema and anaemia commonly found in children with PEM has been suggested to be caused by an imbalance between the production of these toxic radical and their safe disposal [27].
Ascorbic acid or Ascorbate (Vitamin C) is water soluble antioxidants. The human body cannot synthesize it and must be obtained from the diet [28]. Vitamin C has various biological functions involving collagen synthesis, immune function, folate metabolism, iron metabolism, carnitine synthesis and catabolism of cholesterol [29]. It is predominantly absorbed through the distal portion of the small intestine and enters the blood stream. The most widely known complication of vitamin C deficiency is scurvy, a disease marked by an increased incidence of infections and decreased immune response [30]. Vitamin C functions as an antioxidant to protect intracellular and extra cellular component from free radical damage [27], and appears to reduce oxidation mediated damage to DNA in lymphocytes and may enhance production of interleukin-1 and tumor necrosis factor-a. There is paucity of data on the relationship between ascorbic acid and PEM in Sokoto state has been reported among PEM. This aim of this study was to investigate the prevalence of PEM among children in Sokoto, Nigeria and to determine the relationship between PEM and ascorbic acid among children attending Usmanu Danfodiyo University Teaching Hospital (UDUTH) and Specialist Hospital, Sokoto. Result from this study will provide valuable information that will optimize the care offered to children with PEM in the area. It may also justify the need to possibly supplement PEM children in the area micronutrients and vitamins.
Materials and Methods
Study area
The study was conducted at the Paediatrics Department of Usmanu Danfodiyo University Teaching Hospital (UDUTH) and Specialist Hospital, Sokoto State, Nigeria. Both hospitals serve as a referral center for both poor and rich people of Nigerian States of Sokoto, Kebbi and Zamfara and the neighboring Niger and Benin Republic, in the West African sub region (SSBD, 2007). The study area is located in Sokoto State, which is in the extreme North- Western part of Nigeria between longitude 05° 111 to 13° 031 East latitudes 13° 001 to 13° 061 North. The State share borders with the Republic of Niger to the North, Kebbi State to the West and South East and Zamfara to the East. The State covers a land area of about 32,000 square kilometers and with a population of 4.602298 million based on the United Nation Population Fund projection [31]. Sokoto is on a whole, a very hot area. However, maximum daytime temperatures are most of the year generally under 40 °C (104 °F). The warmest months are February to April. The raining season is from June to October during which showers are a daily occurrence, although rarely last long compared to that of the Wet tropical regions. The indigenous inhabitants of the area are the Hausas and Fulani. Other ethnic group resident in the State includes; Igbo, Ebira, Yoruba, Igala as well as Buzus from the neighboring Niger Republic. Farming and crop production are the major occupation of the people living in the study area. The major crops grown in the area includes millet, sorghum, ground nuts, cowpea and tobacco. Livestock reared includes cattle, sheep, goat, donkey, camel, horses and poultry [32].
Study population
The target population for the study included male and female children aged between 6 months 5 years who were admitted to the Paediatrics units of UDUTH and Specialist Hospital, Sokoto. The subjects consisted of 47 children aged 6 months 5 years with a diagnosis of PEM. The control participants consisted of 43 age-matched apparently healthy and well-nourished children attending the immunization clinic prior to immunization. Signed informed consent was obtained from parents and guardians who demonstrated understanding of the study and were willing for their children or ward to be enrolled in the study.
Inclusion criteria
Inclusion criteria included; age (children that are aged 6 months to 5 years), children diagnosed with any form of PEM and willingness of parents and guardians to offer a written informed consent for their children or ward to participate in the study.
Exclusion criteria
Exclusion criteria included; age (children <6 months or > 5 years), children without PEM and children whose parents and guardians refuse to offer a written informed consent for their children or ward to participate in the study.
Study design
The research was a case-control study involving children with various forms of PEM who served as subjects. The control participants consisted of age matched apparently healthy and well-nourished children. Socio-demographic data were collected using a structured interviewer administered questionnaire. Sociodemographic data collected include: age, gender, socio-economic class of the parent, income, educational status and occupation. Quantitative data were obtained by estimating the ascorbic acid (vitamin C) level among the subjects and control participants.
Ethical consideration
Ethical approval for the study was sought and obtained from the Research and Ethical Committee of UDUTH and State Specialist Hospital, Sokoto and permission to carry out the study was given from the respected head of unit.
Informed written consent
Written informed consent for inclusion into the study was obtained from the parents/guardians of the children.
Subject
The study involved children (6 months-5 years) with a diagnosis of PEM based on the Welcome Trust Classification of PEM at the Paediatrics units of UDUTH and Specialist Hospital, Sokoto. The control sample was obtained from apparently healthy and well-nourished children visiting the immunization clinic prior to immunization.
Blood sample collection and processing
Two milliliters (2mls) of venous blood samples was collected ascetically into plain sample bottle for vitamin C estimations. Serum vitamin C (ascorbic acid) was assayed using chemical standard method [33]. The principle of the test is based on the ability of ascorbic acid to be oxidized by copper II ion to form dehydroascorbic acid, which reacts with acidic 2, 4-dinitrophenylhydrazine to form a red bis-hydrazone which is measured spectrophotometrically at 520nm.
Questionnaire
Questionnaire was used to collect bio data and related information from the parents and guardians of the subjects and control participants. A copy of it is attached in the appendix III.
Statistical Analysis
The data analysis was performed using statistical package of social sciences (SPSS) version 22.0. Data was presented as mean ± standard error of mean (SEM) and percentage. Student t-test for mean comparison between two groups was used. Test for association (Chi-square, if appropriate) between categorical variables was used. One-way analysis of variance (ANOVA) with Least Significant Difference (LSD) was employed for mean comparison between the types of PEM. Correlation between the pattern of full blood count and biochemical parameters among children with PEM was done using Pearson’s linear correlation tool. A p-value of less than 0.05 (p≤0.05) was considered as statistically significant in all statistical analysis.
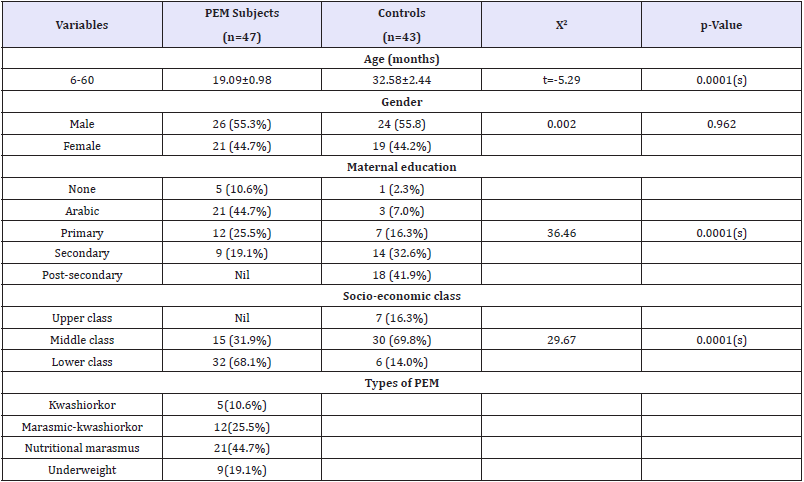
Table 1: The socio-demographic characteristics of subjects and controls.
Result
Subjects for this case-control study included 47 PEM subjects recruited from the Paediatric units of UDUTH and Specialist Hospitals. Forty-three apparently healthy children recruited from the immunization clinics of the same hospitals were monitored as controls. Table 1 shows the socio-demographic characteristics of the PEM subjects and controls. A proportional comparison of mean age, maternal education and parent socio-economic status showed statistically significant difference (p<0.05), while the gender shows no statistical difference (p>0.05). Table 2 revealed that the ascorbic acid levels was significantly lower among PEM subjects as compared to controls (p<0.05). Table 3 shows the pattern of ascorbic acid levels based on the type of PEM. Ascorbic acid levels of underweight subjects were significantly lower compared to other types of PEM. There was no statistically significant difference in the pantothenic acid levels for all the types of PEM, although kwashiorkor recorded the lowest value (Table 1).
Table 2: Mean Comparison of Ascobic Acid Levels among subjects and controls.
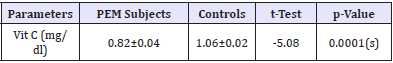
Data are presented as mean ± SEM for age and percentages for others. Figures in brackets are percentages of total, t = t-test, x2 = chi-square, (s) =statistically significant [Table 2].
Table 3: The pattern of ascorbic acid levels based on the type of PEM.
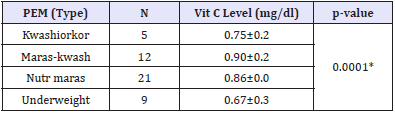
Data are presented as mean ± SEM. PEM= Protein energy malnutrition, Vit C = vitamin C (Ascorbic acid), Vit B5 = vitamin B5 (pantothenic acid), (s)=statistically significant [Table 3].
Data are presented as mean ± SEM. PEM= Protein energy malnutrition, Vit C = vitamin C (Asorbic acid), Maras-kwash = Marasmic-kwashiorkor, Nutr maras = Nutritional marasmus, Grp = Group, Grp l= kwashiorkor, Grp ll= marasmic-kwashiorkor, Grp lll= nutritional marasmus, Grp lV= underweight, *=statistically significant.
Discussion
Protein energy malnutrition (PEM) among preschool children continues to be a major public health problem throughout the developing world. Malnutrition increases one’s susceptibility to and severity of infections, and is the major component of illness and death from diseases. The risk of death is directly correlated with the degree of malnutrition [33]. The children with PEM included in this study were aged 6 months- 5 years. This conforms with previous reports [34-36] in sub Saharan Africa which indicated that a significant number of under five deaths can be attributed to malnutrition. This could be due to a number of factors including low rate of exclusive breast feeding as well as poor weaning and feeding practices [37].
In this study, a high male to female ratio was observed among PEM subjects (1.2:1). This agrees with earlier reviews in Maputo [38,39] but is at variance to the findings of Odebode and Odebode [40] who reported a male to female ratio of 1:1.7 showing a higher incidence of PEM in females compared to males. A high ratio of PEM in male subjects observed in this study might be as a result of higher protein requirement in males than in females, among other unknown reasons.
In this study, maternal level of educational attainment had a great significance (p<0.05) on the incidence of PEM subjects. The incidence of PEM was higher among children whose mothers had no formal education but only Arabic education. This finding is consistent with previous reports [41,42] in Karachi, India and Maiduguri Nigeria respectively. The level of education of a mother can play an important role in the nutritional status of her children. Better educated mothers are more informed about developmental initiatives such as the childhood survival strategies after weaning. The tendency for improper feeding practices and unhygienic preparation of over-diluted formula or starch gruels as regards to the feeding of the children would be very minimal. They are more likely to offer their children good quality and affordable foods to meeting the need of the growing children to meet their protein and/or energy needs.
In this study, the socio-economic class of the parent had a great significance on the nutritional status of the children (p<0.05). The PEM subjects were more from the lower socio-economic class (68.1 %) compared to controls. This observation is supported by previous reports [35-38] that indicated that low socio-economic status of parents is closely associated with the incidence of PEM. The low socio-economic status of parent can result in late visit to hospital for initial treatment of their wards due to their inability to afford the high cost of health care services. Affordability of balanced diet to their growing children can been compromised, they may lack information on highly nutritious but affordable food options in the local environment, live in poor sanitary malaria and other disease prone environment, less likely to have access to clean portable water and toilet facilities [39-41].
In this study, marasmus (44.7%) had higher prevalence followed by marasmic-kwashiorkor (25.5%) among the PEM patients admitted in both UDUTH and specialist hospital. This finding is in agreement with previous reports [37,42,43] which indicated that marasmus is a serious problem and is most common in developing regions such as Africa, Latin America and South Asia, where poverty along with inadequate food supplies and contaminated water are prevalent and that marasmus is most frequently associated with infections. Each year not less than one million Nigerian children die before their fifth birthday. Malnutrition contributes to nearly half of these deaths. In Nigeria, malnutrition remains a great challenge, particularly for mothers and children. PEM is associated with 49% of the 10 million deaths occurring in the developing world and 52% of all under-five deaths in Nigeria [44], with 24% and 16% of the total under-five. Nigerian population is estimated to have suffered from mild-moderate and severe malnutrition respectively from 1973 to 1983 [45].
In this study, we observed that the ascorbic acid levels were significantly lower among children with PEM compared to controls. Our finding is consistent with previous report which indicates that malnourished children have more oxidant damage products and less antioxidant levels [46,47]. Similarly, a previous study indicated that the prevalence of low serum levels of water soluble ascorbic acid was higher (p<0.01) in malnourished elderly than in the non- PEM group (80.9 vs. 56.7) [48].
Similarly, plasma ascorbic acid (AA) levels were estimated in 20 healthy and 80 sick children suffering from various forms of acute illness of less than one week’s duration. The mean plasma AA level in children with acute infective illness who had diet containing inadequate vitamin C content preceding illness was significantly lower compared to the healthy children [49]. There is increasing justification to provide ascorbic acid supplementation for children with PEM [50,51]. Vitamin C an essential nutrient involved in many anabolic pathways. It is also an important player of the endogenous antioxidant defense. Low plasma levels are very common in critical care patients and may reflect severe deficiency states [52]. This justification for ascorbic acid supplementation in PEM seems to be based on the notion that ascorbic acid role in the modulation of oxidative stress and endothelial and protection and thus can potentially offer interesting therapeutic perspectives, based on the biochemical evidence coupled with limited or even absent side-effects. Recent clinical studies reported on the effects of prophylactic administration of antioxidants, as a component of nutritional support to patients at risk for oxidant-related complications [53]. Increased free radical generation and damage in critically ill patients has been associated with greater morbidity and mortality [54]. However, whether the addition of vitamin C supplements would expedite recovery among children with PEM will need further evaluation.
In this study, it was observed that children with underweight had a significantly lower ascorbic acid level when compared with other types of PEM; Kwashiorkor, Maras-kwash and Nutr maras (p<0.05). This observation might be due to poor nutrient amongst others. A significant amount of stress is created as a result of PEM which is responsible for the overproduction of reactive oxygen species (ROSs). This ROSs can potentially lead to membrane oxidation and thus an increase in lipid peroxidation byproducts such as MDA and protein oxidation byproducts such as protein carbonyl mainly. Decrease in level of these antioxidants results in increased oxidant damage. Changes in oxidant and antioxidant levels may be responsible for grading in PEM [55]. Defense capacity against ROS can be measured by determining the blood levels of GSH, glutathione peroxidase (GPx), copper, zinc and ascorbic acid. The pathogenesis of extreme muscle wasting (emaciation) commonly found in children with PEM has been suggested to be caused by an imbalance between the production of these toxic free radicals and antioxidant potential [56].
Study Limitations
The study encountered several limitations including:
1. Refusal of few mothers and guardians to sign the consent form and therefore refusing their children to be included in the study.
2. There was no way to follow- up the PEM subjects from the time of onset of the malnutrition to the time of recovery
3. Nutritional marasmus followed by marasmic-kwashiorkor had the highest prevalence among the PEM subjects. There was a challenge getting other types of PEM.
Conclusion and Recommendations
Findings of this study has shown that PEM subjects had a lower ascorbic compared to controls. The ascorbic acid of children with underweight is significantly lower compared with other types of PEM. Marasmus is the most common type of PEM among the paediatric subjects. There may be need to provide ascorbic acid supplementation for children with PEM [57].
References
- Barker HM, Lees R (1996) Nutrition and dietetics for health care. (9th edn), Churchill Livingstone, UK, pp. 3-10.
- Joshi HS, Joshi MC, Singh A, Joshi P, Israh Khan N (2011) Determination of protein energy malnutrition (pem) in 0-6years children in rural community of bareilly indian. Journal of Preventive & Social Medicine 42(2): 154-155.
- Lawoyin TO (2001) Risk factors for infant mortality in a rural african community in nigeria. Journal of Royal Society for the Promotion of Health 121(2): 114-118.
- National Population Commission (NPC) (2013) Demographic and health survey. Nutrition of Children and Women 11: 175-181.
- WHO (2003) Alleviating protein energy malnutrition. Geneva. World Health Organization, Geneva, Switzerland 30: 5-10.
- Stein TP (2001) Nutrition in the space station era. Nutrition Research Reviews 14(1): 87-117.
- Lane HW, Schulz LO (1992) Nutritional questions relevant to space flight. Annual Review of Nutrition 12: 257-278.
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ (2002) Selected major risk factors and global and regional burden of disease. Lancet 360(9343): 1347-1360.
- Black RE, Morris SS, Bryce J (2003) Where and why are 10 million children dying every year? Lancet 361(9376): 2226-2234.
- UNACC (2000) Fourth report on the world nutrition situation. United Nation Administrative Committee on Coordination/Sub-Committee on Nutrition, Geneva, USA 1(2): 22-27.
- WHO (2000) Nutrition for health and development of global agenda for combating malnutrition, France. World Health Organization, Geneva, Switzerland 41: 55-58.
- Mary EP (2008) Protein energy malnutrition, pathophysiology, clinical consequences and treatment’. In: Walker AW, Christopher D, Watkim JB (eds), Nutrition in Paediatrics. Blackwell Waterson, London, pp. 171-184.
- Hendrickse RT (1991) Protein energy malnutrition. In: Hendrickse RC, Barr DGD, Mathews TS (Eds.), Paediatrics in the Tropics. Blackwell Scientific Publications, London, pp. 119-131.
- Poskitt EME (1988) Protein energy malnutrition in practical pediatrics nutrition. (1st edn), Great Britain Butterworth and Co Ltd, UK, pp. 96- 113.
- Welcome Trust Working Party (1970) Classification of protein energy malnutrition. Lancet 2: 302.
- Passmore R, Eastwood MA (1987) Protein energy malnutrition. Human nutrition and diabetics. (8th edn), Churchill Livingstone, London, pp. 279-310.
- Quereshi MI, Quereshi Z (2001) Effect of protein energy malnutrition on the weight and serum albumin of albino rats. J Ayub Med Coll Abbottabad 13(1): 8-10.
- Cho MK, Kim YG, Lee MGS (1999) Suppression of rat hepatic cytochrome p450 by protein calorie malnutrition- complete or partial restoration by cysteine methionine supplementation. Archives of Biochemistry 372(1): 150-158.
- Den Besten L, Bac M, Glatthraar II (1995) Changes in the anthropometric status of rural african under-five, during a decade of primary health care. Journal of Tropical Medicine and Hygiene 98: 361-368.
- Oyedeji GA (2000) Is it well with the nigerian child? Obafemi Awolowo University Ile Ife, Nigerian Inaugural Lecture Series 171: 13.
- Bamgboye EA, Familusi JB (1990) Morbidity trends at the children’s emergency room university college hospital ibadan, Nigeria. African Medical Journal 19(1): 49-56.
- UNICEF (2000) Strategy for improved nutrition of children and women in developing countries. United International Children Emergency Fund, New York, USA, p. 12.
- Federal Office of Statistics (1990) Nutritional status. National Demographic and Health Survey. Nigeria 9(2): 112-115.
- (2013) Nigeria demographic and health survey (NDHS).
- Uma S, Thankappan KP, Sarma PS (2001) Assessing potential risk factors for child malnutrition in rural keral india. Journal of Tropical Pediatrics 47(6): 350-355.
- Khan MR, Rahman ME (2011) Nutritional problem. Essence of paediatrics. (4th edn), 5: 72.
- Ebrahim GJ (2016) Vitamins in health and disease: Mother and child nutrition in the tropics and subtropics. Journal of Tropical Pediatrics 8: 326-334.
- Ashour MN, Salem SI, El Gadban HM, Elwan NM, Basu TK (1999) Antioxidant status in children with protein-energy malnutrition (PEM) living in Cairo, Egypt’. European Journal of Clinical Nutrition 53(8): 669-673.
- Sminoff N (2001) L-ascorbic acid biosynthesis. Vitam Horm 61: 241- 266.
- Brennan LA, Morris GM, Wasson GR, Hannigan BM, Barnett YA (2000) The effect of vitamin C or vitamin E supplementation on basal and H2O2-induced DNA damage in human lymphocytes. British Journal of Nutrition 84(2): 195-202.
- United Nation Population Fund (UNFPA) (2013) Annual report: Population projection and health services in sokoto state, Nigeria.
- Natelson S (1971) Techniques of clinical chemistry, (3rd edn), Thomas CC, USA 162: 288.
- Fernandex ID, Himes JH, De Oris M (2002) Prevalence of nutritional wasting in populations: building explanatory models using secondary data. Bull World Health Organ 80(4): 282-291.
- Yusuf T, Jiya NM, Ahmed H, Baba J, Haruna AS (2014) Prevalence of HIVinfection among under-5 children with protein energy malnutrition presenting at Usmanu Danfodiyo University Teaching Hospital, Sokoto, Nigeria. Nigerian Journal of Paediatric 41(4): 341-344.
- Muoneke V, Ibekwe RU, Nebe Agumadu H (2012) Factors associated with mortality in under-five children with severe anaemia in ebonyi, nigeria. Journal of Indian Paediatric 49(2): 119-123.
- Saka AO, Saka MJ, Ojuawo A, Abdulkarim A, Bilamin S, Latubosun L, Adeboye M (2012) Haematological profile in children with protein energy malnutrition in North Central Nigeria. Global Journal of Medical Research 12(4): 1-7.
- Ubesie AC, Ibeziako NS, Ndiokwelu CI, Uzoka CM, Nwafor CA (2012) Under-five protein energy malnutrition admitted at the university teaching hospital of Enugu in Nigeria: a 10 years’ retrospective review. Nutritional Journal 11: 43.
- Cartmell E, Natalal H, Ferreira MH, Grahnquist L (2005) Nutritional and clinical status of children admitted to the malnutrition ward, Maputo central hospital: Comparison of data from 2001 and 1983. Journal of Tropical Paediatric 51(2): 102-105.
- Odebode TO, Odebode SO (2005) Protein energy malnutrition and nervous system: the impact of socioeconomic condition, weaning practice, infection and food intake, an experience in Nigeria. Pakistan Journal of Nutrition 4(5): 304-309.
- Ali SS, Karim N, Billoo AG, Haider SS (2005) Association of literacy of mothers with malnutrition among children under three years of age in rural area of district Malir, Karachi. Journal of Pakistan Medical Association 55(12): 550-553.
- Basheir HM, Hamza MK (2015) Hematological parameters of malnourished sudanese children under-five years, khartoum state 2011. Clinical Medicine Journal 1(4): 152-156.
- Oyedeji GA (2000) Is it well with the Nigerian child? Obafemi Awolowo University Ile Ife, Nigerian Inaugural Lecture Series 171: 13.
- Khare M, Mohanty C, Das BK, Jyoti A, Mukhopadhyay B, et al. (2014) Free radicals and antioxidant status in protein energy malnutrition. International Journal of Pediatrics 2014: 254396.
- Ashour MN, Salem SI, El Gadban HM, Elwan NM, Basu TK (1999) Antioxidant status in children with protein-energy malnutrition (PEM) living in Cairo, Egypt. European Journal of Clinical Nutrition 53(8): 669-673.
- Cunha DF, Cunha SF, Unamuno MR, Vannucchi H (2001) Serum levels assessment of vitamin A, E, C, B2 and carotenoids in malnourished and non-malnourished hospitalized elderly patients. Clin Nutr 20(2): 167- 170.
- Shivpuri S, Saxena S (1982) Plasma ascorbic acid levels in children with acute illness. Indian Journal of Pediatrics 49(6): 129-133.
- Gupta M, Agarwal KN (1972) Blood vitamin C levels in healthy and malnourished preschool children. Indian Pediatrics 9: 220.
- Abilés J, de la Cruz AP, Castaño J, Rodríguez Elvira M, Aguayo E, et al. (2006) Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: a cohort study. Critical Care 10(5): R146.
- Berger MM, Oudemans van Straaten HM (2005) Vitamin C supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care 18(2): 193-201.
- Bulger EM, Maier RV (2001) Antioxidants in critical illness. Arch Surg 136(10): 1201-1207.
- Roth E, Manhart N, Wessner B (2004) Assessing the antioxidative status in critically ill patients. Current Opinion in Clinical Nutrition and Metabolic Care 7(2): 161-168.
- Khare M, Mohanty C, Das BK, Jyoti A, Mukhopadhyay B, et al. (2014) Free radicals and antioxidant status in protein energy malnutrition. International Journal of Pediatrics 2014: 254396.
- Jimoh FO, Odutuga AA, Oladiji AT (2005) Status of lipid peroxidation and antioxidant enzymes in the tissues of rats fed low-protein diet. Pakistan Journal of Nutrition 4(6): 431-434.
- Bulger EM, Maier RV (2001) Antioxidants in critical illness. Archives of Surgery 136(10): 1201-1207.
- Preiser JC, Gossum AV, Berré J, Vincent JL, Carpentier Y, et al. (2000) Enteral feeding with a solution enriched with antioxidant vitamins A, C and E enhances the resistance to oxidative stress. Crit Care Med 28(12): 3828-3832.
- Khare M, Mohanty C, Das BK, Jyoti A, Mukhopadhyay B, et al. (2014) Free radicals and antioxidant status in protein energy malnutrition. International Journal of Pediatrics 2014: 254396.
- Ashour MN, Salem SI, El Gadban HM, Elwan NM, Basu TK (1999) Antioxidant status in children with protein-energy malnutrition (PEM) living in Cairo, Egypt. European Journal of Clinical Nutrition 52: 669- 673.
© 2018 Erhabor O. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






