- Submissions

Full Text
COJ Biomedical Science & Research
In-Vitro Bioactivity of Microalgal Extracts
Ozcan DO* and Ovez B
1Chemical Engineering Department, Turkey
*Corresponding author: Ozcan DO, Chemical Engineering Department, Turkey
Submission: July 11, 2020; Published: November 16, 2020

Volume1 Issue3November 2020
Abstract
In recent years, the issue of bioactive molecules derived from microalgae has emerged as important sources that can be used in human and animal nutrition, as well as their antibiotic, antiviral, anticancer, antifungal, antibacterial, anti-inflammatory effects. These natural compounds cannot be only assigned as pharmaceutical raw materials, but also in the construction of synthetic molecules. On the other side when compared with the classic ingredients of the food industry; the demand for the products that contain one or more functional compounds with health care contribution is rapidly increasing. According to this fact, market shares of the natural products such as flavonoids, catechins, phenols, and phenolic acid have reached to a point that cannot be ignored. In the literature, the bioactivity of the natural products for the effects of the immune system on acute or chronic reactions of the stimulants as injury, inflammation have been investigated and this paper includes a mini review on in-vitro bioactivity of microalgal extracts together with the total phenolic contents and antioxidant capacities.
Keywords: Antioxidant; Bioactivity;Microalgae; Phenol
Introduction
Microalgae are planktons, of which the sizes vary from a few microns to several hundred microns, and basic producers that synthesize the complex molecules necessary for their life via photosynthesis with carbon dioxide and inorganic substances in their cells with the help of chlorophyll mainly in the aquatic environment. The classification of phytoplanktonic microalgae are realized according to their features such as cell morphologies, cytology, pigment substances they contain, and reproduction form they show. Planktonic microalgae groups belonging to Cyanophyta, Chlorophyta, Euglenophyta and Diyatomophyceae cultures are abundant in fresh water, while Pyrrhophyta, Crysophyceae, Xanthophyceae cultures are relatively less. The most common group found in the brackish water is the Diyatomophyceae with its many species. Studies on the cellular structures, metabolism, and adaptation mechanisms of microalgae, which constitute a very important part of aquatic organisms and accepted as the first step of life, have been increasing in recent years. Exposing microalgal cultures to stress conditions in cultivation environments causes their metabolism to alter and makes them advantageous in many application areas. At this stage, determining the reproductive efficiency of microalgae and expressing them with mathematical models constitutes the sustainability criterion of the system. In particular, different studies have been done on modeling the effect of light intensity and temperature variables.
Microalgae are grown for the production of high value-added bioproducts having many bioactivities as well as energy raw materials, and high protein content playing an important role in feeding of animals in rivers and seas and thereby contributing to a more sustainable bio-sourced economy. Because of the protein, carbohydrates, fatty acids, vitamins, mineral pigments, and many other important products they accumulate in their cells, they are also used as a nutritional supplement by humans. It is important to obtain and expand the industrial use of these products of natural origin, such as flavonoids, catechins, phenols (carnosol, rosmanol, rosamaridifenol) and phenolic acid (carnosic acid, rosmarinic acid), which can be used commercially in the food and pharmaceutical industry [1].
The presence of some important phenolic components (ascorbic acid, cinnamic acid, salicylic acid, gallic acid, caffeic acid, chlorogenic acid, vanillic acid, syringic acid, gentisic acid, ferrulic acid, sinapic acid, p-coumaric acid, protocatechuic acid, 2,3-dihydroxybenzoic acid, kaempferol, catechin, epicatechin, epigallocatechin) were investigated by Farvin & Jacobsen [2] from 16 different seaweed, Benayad et al. [3] from cactus flowers, Hyun et al. [4] from Dendropanax morbifera leaves, Klejdus et al. [5] from Cyanobacteria sp., Machu et al. [6] from many different algae species extracts.
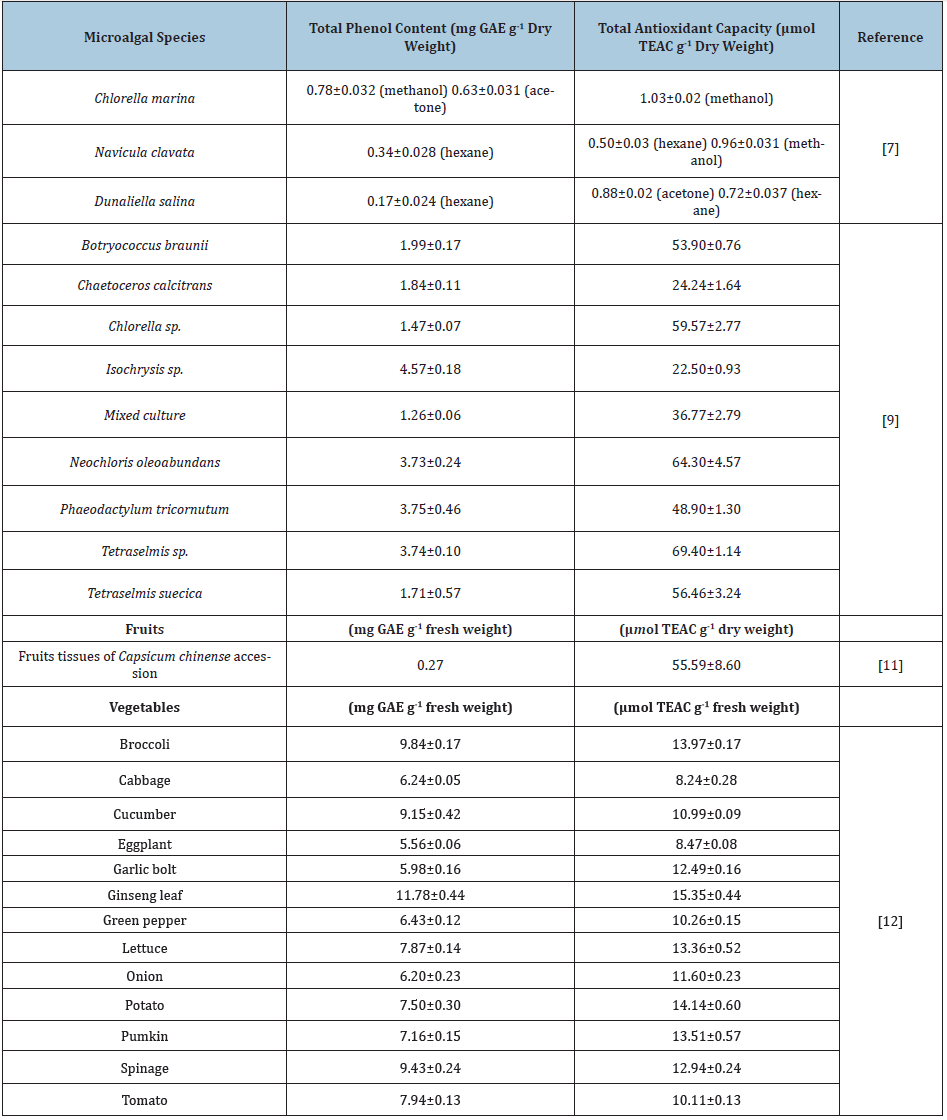
In studies conducted with algal biomass throughout the literature, it has been observed that the total phenol, total antioxidant, and total flavonoid contents were examined. The results of the analyzes made with biomass or extracts vary according to the conditions they were obtained. Hemalatha et al. [7] used three different solvents (methanol, acetone, and hexane) in the study with Chlorella marina, Navicula clavata, and Dunaliella salina microalgae as tabulated in Table 1. It is clearly seen that both the phenolic and antioxidant content varied according to the extraction solvents. Miranda et al. [8] found that in their study with Chlorella vulgaris grown under special conditions, they found the content of this species as 24.95mg Catechin per g dry weight. In the study of Goiris et al. [9], the carotenoid, phenolic contents of phenolic substances and antioxidant activities in ethanol/water extracts from different microalgal biomass were stated in Table 1. It was concluded that the phenolic content in the ethanol/water extracts varied from 1.26 to 4.57mg GAE per g dry weight where relatively high phenolic contents greater than 3mg GAE per g dry weight belonged to Isochrysis, Neochloris oleoabundans, Phaeodactylum, Tetraselmis. and a mixed culture of marine diatoms [9]. Similarly, in Table 1, the antioxidant capacity varied depending on the species with a maximum value of 69.40μmol TEAC per g biomass in ethanol/water extracts where relatively high phenolic contents greater than 40μmol TEAC per g dry weight belonged to Botryococcus braunii, Chlorella, Neochloris oleoabundans, Phaeodactylum tricornutum, Tetraselmis and Tetraselmis suecica [9]. In another study with Chlorella protothecoides, Chlorella pyrenoidosa, Chlorella vulgaris, Chlorella zofingiensis and Nitzschia laevis, the total amount of phenol was given as 14.95, 13.90, 11.43, 3.47 and 2.37µg GAE per g dry weight where the total antioxidant capacity was 11.41, 8.4, 5.53, 1.83 and 2.21µmol TEAC per g dry weight [10-12]. Both total phenolic content and antioxidant capacities of microalgal species are compared with some fruits and vegetables and presented in Table 1.
Table 1: The total phenol contents and antioxidant activities of different microalgal species studied in the literature in comparison with some fruits and vegetables.
Conclusion
The structural profile of the microalgae, the cultivation conditions and the solvent used for the extraction process all contribute to the determination of the in-vitro bioactivity of microalgal extracts. A mini review has been made that examines the bioactivities of the microalgal species in the literature. In this aspect, it is important to design a study that has commercial, economic, and social outcomes.
References
- Chandini SK, Ganesan P, Bhaskar N (2008) In vitro antioxidant activities of three selected brown seaweeds of India. Food Chemistry 107: 707-713.
- Farvin KH, Jacobsen C (2013) Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chemistry 138: 1670-1681.
- Benayad Z, Villaluenga CM, Frias J, Cordoves CG, Safi N (2014) Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Industrial Crops and Products 62: 412-420.
- Hyun TK, Ko YJ, Kim EH, Chung M, Kim JS (2015) Anti-inflammatory activity and phenolic composition of Dendropanax morbifera leaf extracts. Industrial Crops and Products 74: 263-270.
- Klejdus B, Kopecky J, Benesova L, Vacek J (2009) Solid-phase/supercritical-fluid extraction for liquid chromatography of phenolic compounds in freshwater microalgae and selected cyanobacterial species. Journal of Chromatography A 1216: 763-771.
- Machu LJV, Orsavova J, Mlcek J, Sochor J, Jurikova T (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20: 1118-1133.
- Hemalatha A, Girija K, Parthiban C, Saranya C, Anantharaman P (2013) Antioxidant properties and total phenolic content of a marine diatom, Navicula clavata and green microalgae, Chlorella marina and Dunaliella salina. Advances in Applied Science Research 4: 151-157.
- Miranda MS, Sato S, Filho JM (2001) Antioxidant activity of the microalga Chlorella vulgaris cultered on special conditions. Boll Chim Farm 140: 165-168.
- Goiris K, Muylaert K, Fraeye I, Foubert I, Brabanter J, et al. (2012) Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. Journal of Applied Phycology 1: 1-10.
- Lia H, Cheng K, Wong C, Fana K, Chena F, et al. (2007) Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chemistry 102: 771-776.
- Concha CLA, Che TJ, Mukul MA, Flota VFA, Ham MML (2014) Antioxidant capacity and total phenolic content in fruit tissues from accessions of Capsicum chinense (Habanero Pepper) at different stages of ripening. The Scientific World Journal 2014: 1-5.
- Deng GF, Lin X, Xu XR, Gao LL, Xie JF, et al. (2013) Antioxidant capacities and total phenolic contents of 56 vegetables. Journal of Functional Foods 5: 260-266.
© 2020 Ozcan DO. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






