- Submissions

Full Text
Aspects in Mining & Mineral Science
Nano-Nickel/Porous Silicon-Based Ammonia-Fed Fuel Cells
Dzhafarov TD1*, Bayramov AH1, Ragimov SX1, Bagiyev EA1, Jalilli JN1, and Amiraslanov IR1,2
1Institute of Physics, Ministry of Science and Education of Republic of Azerbaijan, Azerbaijan
2Baku State University, Azerbaijan
*Corresponding author:Tayyar Dzhafarov, Institute of Physics, Ministry of Science and Education of Republic of Azerbaijan, Baku, Azerbaijan
Submission: March 11, 2024: Published: March 27, 2024

ISSN 2578-0255Volume12 Issue3
Abstract
In this paper the results on fabrication technology, structural, electrical characteristics of Nickel/Porous Silicon/Silicon (Ni/PS/Si) structures as direct Ammonia Solution (AS) fuel cells are reported. Here nano-porous silicon acts as proton conducting membrane, Ni as a catalyst layer with the thickness of 12-110nm in AS/Ni/PS/Si fuel cells. X-Ray Diffraction (XRD), electrical measurements and performance characteristics of AS/Ni/PS/Si cells were studied at room temperature. Generation of electricity was observed for Ni//PS/Si cells placed in Ammonia Solution (AS) in the range 0- 25%. Sharp rise of maximum power density (0.7mW/cm2) with the open-circuit voltage V=0.76V and the short-circuit current density J=2.4mA/cm2, when nickel thickness decreases down to 12nm, was observed. Possible reason of this phenomenon is discussed on the basis of quantum size effect which may occur at ultrathin thickness of the catalyst..
Keywords:Nano-nickel/porous silicon fuel cell; Ammonia electrolyte; Maximum power density; Open-circuit voltage
Introduction
A fuel cell is an electrochemical device (a galvanic cell) which converts the energy of a chemical reaction into electricity. It usually consists of two electrodes, a negative electrode (or anode) and a positive electrode (or cathode) sandwiched around an electrolyte. The molecular hydrogen on the catalyst anode is dissociated into protons and electrons, stimulating difference of potentials between anode and cathode, and allows extracting the electricity. Several types of fuel cells, depending on the compound used as fuel are now under development, having own advantages and disadvantages. It seems attractive to use pure hydrogen as a fuel, because the final products of the reaction are heat and water/steam. But the direct use of hydrogen as a fuel meets some difficulties. Moreover, hydrogen has a low energy density in comparison with other hydrogen-containing compounds such as metal hydrides, ammonia, methanol, hydrocarbons and etc.
Polymer Electrolyte Membrane (PEM) cells, known as proton exchange membrane cells, are now considered the leading for automotive applications. PEM fuel cell is a thin, flat, multi-layered “sandwich” and is made up of two electrodes (anode and cathode) surrounding by polymer electrolyte [1]. The porous silicon wafer filled with acid or Nafion® was developed as a proton conducting membrane [2]. Porous silicon due to its original structural properties is attractive to be used in fuel cell structures as a solid “electrolyte”. The crystalline structure of porous silicon presents a network of silicon in nano (micro)-regions with an extremely large surface/volume ratio (up to 800m2/cm3). Existence of pores, especially the canal-form pores, which determine large ion (proton) conductivity opens new perspectives for using porous silicon-based structures as a hydrogen fuel cell [3,4]. The porous surface is covered by Silicon Hydrides (Si-H) and Silicon Oxides (Si-O) bond along which protons can easily move and may play the role of proton-conducting electrolyte in porous silicon-based fuel cells.
Traditionally, platinum group metals (Pt, Pt-Ru, Pt-Ir alloy, RuO2 and Rd) have been employed as the most effective catalysts with most of low temperature fuel cells including those with polymer electrolytes [5]. The membranes in these fuel cells become highly acidic during operation, demanding the use of corrosion resistant construction materials. However, the high cost of platinum group limits of wide use in technology of fuel cells. Many non-noble metals (such as Ni, Fe, Co, Co etc.) based catalysts have been reported [6]. Among them, Ni-based are considered as one of the most promising catalysts for NH3 decomposition, due to their superior catalytic activity, significant price advantage and long-term stability [7]. Nickel is an attractive metal for electrochemical application because of its cheapness and catalytically activity towards many reactions. Nickel-based materials (metallic nickel, its alloys, oxides, hydroxides and composites) have been also considered as promising electrocatalysts for ammonia oxide [8]. Ammonia solution contains of 17% hydrogen by weight, which can be extracted via thermal catalytic decomposition or electro-oxidation [9]. Ammonia’s energy density is (13.6GJ/m3) much higher than that of hydrogen (3.6GJ/ m3) and comparable to that of Compressed Natural Gas (CNG) and methanol. Previous research on porous silicon membrane fuel cells have been focused on Hydrogen, Hydrogen Sulphide, Ammonia [10- 12] et.al. In this paper we report the fabrication details, structural properties, performance characteristics of new type direct porous silicon structure AS/nano Ni/PS/Si, using nano-nickel catalyst and ammonia solution.
Experimental Procedure
Porous silicon layers with thickness of 10-40μm and average porosity from 40% to 80% were prepared on n-type monocrystalline (111) Si substrates with resistivity of 120Ω∙cm by anodic etching in hydrofluoric-ethanol solution under the white light illumination [10]. For some measurements the PS films were then detached from Si substrate by electro-polishing. The electrical measurements of the free-standing PS layers with 65% porosity ended up with ρ=1.8x104Ω.m for resistivity, p=9.6x1018m-3 hole concentration, and μ=3.6x10-5m2/V.s for hole mobility. The Ni/PS/Si n-type structures were fabricated by evaporation of a thin Ni catalyst films onto the PS/Si structure at room temperature by using Mo bout in vacuum of 1.3×10−3Pa. The thicknesses of the deposited nickel films (12nm, 30nm, 50nm, 110nm) were controlled during the deposition process by Inficon. The surface film of nickel differs from volume body, not at all of chemical bonds are active with neighbouring atoms, i.e. for surface atoms are existence of no-saturate bonds. In consequence in the layers are arise strongly distorting of crystallize lattice, change of parameter of lattice and even may take place change of the lattice. In case of using nickel, as nano-catalyst character of thickness a few nanometers, may waiting that distance between neighbour atoms of surface decrease. Thickness of nanonickel catalyst was less than ordinary catalyst (quantum size effect) [10].
X-Ray Diffraction (XRD) measurements of nickel films were performed at room temperature (Bruker XRD D2 PHASER Diffractometer, Germany), using CuKα radiation, in the 2θ range of 5° to 80° at 2°/min. The crystallite size of the Ni catalysts group was derived using the Scherrer equation:
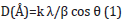
Where k is a coefficient equal to 0.9, λ is the wavelength of X-rays used (1.54056Å), β is the full width at half-maximum of the respective diffraction peak, and θ is the angle at the position on the peak maximum. Еlectric measurements of fuel cells were carried out using ammonia solution (NH3:H2O) of concentration (25%). The composition of the ammonium solution changed with the addition of water. Ammonia and water form ammonium hydroxide on the surface of nickel:
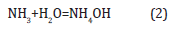
The current-voltage characteristics, open-circuit voltage (Voc) and short-circuit current density (J) of AS/Ni/PS/Si were measured at air ambient (40% RH) directly by digital multi-meter (Thurlby-1503). The measurements were performed at room temperatures between the contacts on NH3 and Si substrate in the dark and daylight. All the cells exhibited weak photosensitivity and therefore ammonia-stimulated measurements of current-voltage characteristics were performed under daylight conditions.
Result and Discussion
Figure 1 illustrates the diffraction patterns of Ni/PS/Si structures with catalysts nickel of 12nm, 30nm, 50 nm and 110nm. XRD patterns of the samples Ni/PS/Si with 50nm and 110nm nickel films significantly differ from the patterns of the samples with 12nm, 30nm Ni. For the samples with 50nm and 110nm cubic structures of Ni with the lattice parameters a=3.5172Å and a=3.5194Å along the direction (111), respectively, are observed. Corresponding grain sizes of Ni crystallites, calculated from the equation (1) are 227Å and 285Å, respectively. Ni/PS/Si structures with 30nm nickel exhibits very weak reflection pattern at the same 2θ position. No reflections were observed in the Ni/PS/Si fuel cell with 12nm nickel. Consequently, Ni layers in the sample with 12nm nickel have a disordered (amorphous) structure. Obviously, such a structure is associated with very small grain sizes because of low thickness and small ionic radius of Ni+2 (0.083nm).
Current density-voltage and current density-power density characteristics of AS/Ni/PS/Si cells with nickel catalysts thickness of d=110nm (curves 1a and 1b) and d=12nm (curves 2a and 2b) are shown in Figure 2, respectively. The same characteristics for AS/ Ni/PS/Si cells with nickel catalyst thickness of d=50nm (curves 1a and 1b) and d=30nm (curves 2a and 2b), respectively are given in Figure 3. Nickel catalyst thickness dependence of maximum power density generation in AS/Ni/PS/Si fuel cells is given in Figure 4.
As it is seen from the Figure 4, sharp increase of the maximum power density of AS/Ni/PS/Si fuel cell is observed (0.7mW/cm2) when was reached at minimum of nano-nickel catalyst (d=12nm) (Table 1).
Figure 1:XRD patterns of Ni on PS/Si with 110, 50, 30 and 12nm nickel films.

Figure 2:Current density-voltage and current density-power density characteristics of AS/Ni/PS/Si cells with nickel catalysts thickness of d=110nm (curves 1a and 1b) and d=12nm (curves 2a and 2b).

Figure 3:Current density-voltage and current density-power density characteristics of AS/Ni/PS/Si cells with nickel catalyst thickness of d=50nm (curves 1a and 1b) and d=30nm (curves 2a and 2b).

Figure 4:Maximum power densities vs. Ni-catalyst thickness of AS/Ni/PS/Si fuel cells.

Table 1:The results on fuel cell parameters are summarized.

The mechanism of the generation of the electricity in the metal/ PS/Si cells under hydrogen-containing ambient has already been proposed [10]. We suggest that in Ni/PS/Si cell, the Ni film and PS layer filled with ammonia play the role of catalytic anode and electrolyte, respectively. The interface region between the porous and crystalline silicon (PS/Si), which is very imperfect and stressed, plays the role of the cathode. The following reactions take place in the presence of a catalyst [13].
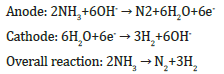
Electrons and hydrogen protons formed in the Ni catalyst film after hydrogen splitting, electrons pass through external circuit reach the cathode (PS interface) region. The oxygen from air can easily penetrate into the PS/Si interface due to the imperfections in this area. Here the hydrogen is recombined and reacts with oxygen to produce water molecules. For the surface atoms of the thin nickel film (12nm), unlike the bulk one, not all chemical bonds are involved with neighbouring atoms, i.e. the surface atoms are characterized by the existence of unsaturated bonds. As a result, a strong distortion of the crystal lattice in the surface and surface layers, i.e. a change in the lattice parameter up to change in the lattice type may take place (see, Figure 1). If nickel is used as a nano-catalyst, characterized by the thickness of tens of nanometers, the distance between surface atoms can be decreased and therefore less H+ protons will pass through the nano-nickel catalyst. In other words, in AS/Ni/PS/Si, at the lower nickel thickness (12nm) as compared with the higher nickel thickness the size effect should be observed. As a result, the dissociation reaction of hydrogen atoms to proton and electron on the surface area of nano-nickel will be enhanced, which leads to increase of output parameters of the fuel cells.
Conclusion
Results of preparation and characterization of novel ammonia fuel cells using porous silicon as a proton conduction membrane, nickel as a nano-catalyst and ammonia solution as a fuel are presented. It is shown that maximum power density of AS/Ni/PS/Si fuel cell at 12nm Ni thickness reaches 0.70mW/cm2, in comparison with 0.12mW/cm2 at 110nm Ni thickness. Drastic increase of the maximum power density of AS/Ni/PS/Si fuel cell, observed below nickel catalyst thickness of d=20nm can be explained by quantum size effect, which may occur at ultrathin thickness of the Ni catalyst.
References
- Dzhafavov TD, Sureyya AD (2011) Nano-porous silicon-based mini hydrogen fuel cells. In: Mahzanera M (Ed.) Alternative Fuel. Intechweb Org, Croatia, pp. 309-334.
- Guoxiao X, Zhiguarg W, Zenglv W, Junli W, Jin Y, et al. (2020) Non-destuctive fabrication of nafion/silica composite membrane via swelling-filling modification strategy for high temperature and humidity PEM fuel cell. Renewable Energy 153: 935-939.
- Gyoko N, Naohiro I, Takaharu T, Jing Rong Y, Koji T, et al. (2005) Porous silicon as a proton exchange membrane for micro fuel cells. Electrochemistry 73(11): 939-941.
- Dzhafarov TD, Bayramov AH (2018) Porous silicon and solar cells. Handbook of Porous Silicon, pp. 1479-1492.
- Okonkwo PC, Ige OO, Barhoumi E, Uzomo P, Emori W, et al. (2021) Platinum degradation mechanisms in proton enhance membrane fuel cell (PEMFC) system: A review. Inter J Hydrogen Energy 46(29): 15850-15865.
- Luezak J, Lieder M (2023) Nickel-based catalysts for electrolytic decomposition for ammonia towards hydrogen production. Advances in Colloid and Interface Science 319: 102963.
- Bell TE, Torrente-Mureino L (2016) H2 production via ammonia decomposition ion used non-noble metal catalysts: A Review. Top Catal 59: 1438-1457.
- Zhou L, Znang T, Tao Z, Chen J (2014) Ni nanoparticles supported on carbon as efficient catalysts for the hydrolysis of ammonia borans. Nano Research 7: 774-781.
- Zamfirescu C, Dincer I (2008) Using ammonia as sustainable fuel. J Power Sources 185(1): 459-465.
- Dzhafarov TD, Oruc C, Aydin S (2004) Humidity-voltaic characteristics of au-porous silicon interface. J Phys D Appl Phys 37: 404-408.
- Dzhafarov TD, Aydin YS (2011) Porous silicon-based direct hydrogen sulphide fuel cells. Nanoscience and Nanotechnology 11(10): 9012-9015.
- Dzhafarov TD, Bayramov AH, Ragimov SX, Bagimov EA, Asadullayeva SG, et al. (2023) Nano-nickel as a catalyst for the Ni/PS/Si/hydrogen fuel cells. Aspects in Mining and Mineral Science 11(2): 1222-1225.
- Basu S (2007) Fuel science catalysts and technology. Springer, New York, USA, p. 40.
© 2024 Tayyar Dzhafarov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






