- Submissions

Full Text
Research in Medical & Engineering Sciences
Synthesis, Characterization, Antimicrobial and Antioxidant Assay of Costus Igneus Bio-Active Compounds Loaded Zinc Nanoparticles for Nano and Bioactive
Thiruchenduran S1*, Supraja N2 and Prasad TNVKV2
1National Aquatic Resources Research and Development Agency (NARA), Sri Lanka
2Acharya n g Ranga Agricultural University, India
*Corresponding author: Thiruchenduran S, National Aquatic Resources Research and Development Agency (NARA), Crow Island, Colombo 15, Sri Lanka
Submission: June 02, 2018; Published: July 02, 2018

ISSN: 2576-8816
Volume5 Issue5
Abstract
Nanodelivery of bioactive compounds carried on nanoparticles has become a wide spread application in agro, food and biomedical research in the recent past. The aim of the study is to mount bioactive compounds onto Zinc oxide nanoparticles (ZnNP) and assess its properties. The ZnNPs were synthesized in bottom-up approach using Costus igneusbioactive compounds (CIBC). ZnNPs were characterized for nanoproperties that are surface plasmon resonance (SPR), particle size distribution and zetapotential. Bioactive properties of CIBC loaded ZnNP was evaluated using FT-IR, antimicrobial assay and antioxidant assay. Antifungal activity of the CIBC loaded ZnNPs were assessed for fungal species Aspergillus niger, Sclerotium sp, Rhizophus rolfzi, Aspergillus flavusagainst positive controls of 30μg 5mm discs of itraconazole (IZ) and ketoconazole (KZ). Antibacterial activity of CIBC loaded ZnNPs was assessed against bacteria species Bacillus subtilis, Streptomyces sp, Pseudomonas fluorescence and Staphylococcus aureususing disc diffusion methodand the results were compared with positive controls of 30μg 5mm discs of tetracycline (TCN), ampicillin (ACN), penicillin (PCN) and CMZ. SPR of the formed ZnNPs were recorded at 260nm using UV-Visible spectrophotometer. The average ZnNP particle size was 42.3nm at 90° scattering angle with monodisperse mode. Three different concentration of the ZnNP colloids were used (170ppm, 100pp and 80ppm). High zetapotential of -16.3mv at 25°C and a conductivity of 0.463ms/cm indicated the high stability of the nanoparticles. The presence of functional groups poly alcohols, carboxylic acids and benzene rings attached to the ZnNPs were identified using FT-IR analysis. CIBC loaded ZnNPs exhibited significant (p<0.05, Tukey’s HSD test) antifungal activity at 170ppm against Aspergillus nigerand Aspergillus flavus significant (p<0.05, Tukey’s HSD test) antibacterial activity at 170ppm against Streptomyces sp, Pseudomonas fluorescenceand Staphylococcus aureus. CIBC loaded ZnNPs showed 0.23μmol/ml trolox equivalents DPPH radical scavenging activity at 170ppm. Thus the results showed the significant potentials of CIBC loaded ZnNPs in biological applications as antimicrobial agents and bioactive compound carriers.
Keywords: Nanoparticles; Zinc; Costus igneus; Antimicrobial activity; Anti-oxidant assay
Introduction
Nanomaterials are proposed to be the materials for the new millennium. Metal nanoparticles (1-100nm) have various functions that are not observed in bulk phase and have been studied extensively because of their exclusive catalytic, optical, electronic, magnetic, antimicrobial wound healing and anti-inflammatory properties [1]. Nanomaterials exhibit unique and considerably changed physical, chemical and biological properties when compared to their bulk counterparts [2].
Metal nanoparticles are intensely studied due to their unique optical, electrical and catalytic properties. To utilize and optimize chemical or physical properties of nano-sized metal particles, a large spectrum of research has been focused to control the size and shape, which is crucial in tuning their physical, chemical and optical properties. Various techniques, including chemical and physical means have been developed to prepare metal nanoparticles, such as chemical reduction, electrochemical reduction, photochemical reduction, heat evaporation and so on. In most cases, the surface passivator reagents are needed to prevent nanoparticles from aggregation. Biosynthesis of nanoparticles has received considerable attention due to the growing need to develop environmentally benign technologies in material synthesis. For instance, a great deal of effort has been put into the biosynthesis of inorganic materials, especially metal nanoparticles using microorganisms. Both live and dead microorganisms are gaining importance by virtue of their facile assembly of nanoparticles. Moreover, the problems concerning the synthesis of nanoparticles and their stabilization can be solved in tandem and mild conditions.
Use of plant extract for the synthesis of nanoparticles could be advantageous over other environmentally benign biological processes by eliminating the elaborate process of maintaining cell cultures. Green synthesis of metal nanoparticles from plants is an interesting aspect as the process is eco-friendly, and nontoxic. Metal nanoparticles are successfully synthesized by green synthesis route.
Costus igneus is a commonly known as insulin plant in India, belongs to family Costaceae herbaceous plant with a range of biological activities including anti-diabetic, anti-hypertension, antiinflammatory properties which can be attributed to the presence of different classes of bioactive compounds [3]. It is believed that consumption of the leaves helps lower the blood glucose levels, and diabetics who consumed the leaves of this plant report a fall in their blood glucose levels. This study was planned to evaluate the hypoglycemic effect of Costus igneus, on dexamethasone-induced hyperglycemia.
The aim of the study is to synthesis of ZnNPs loaded with CIBC and assess their nano and bioactive properties via successfully synthesizing nanoparticles, characterizing the ZnNP for their nanoproperties, identifying the presence of CIBC on the ZnNPs, assessing the antimicrobial activities of the CIBC loaded ZnNPs and assessing the antioxidant capacities of the nanoparticles.
Materials and Methodology
Chemicals
Zinc nitrate (99 % pure) was purchased from Sigma Aldrich, India. Potato dextrose broth, Potato dextrose agar, Nutrient broth, and Nutrient agar plate were supplied by Hi-media, India.
Collection of Costus igneus leaf samples
Costus igneus leaves were collected from the herbal garden maintained by Sri Konda Lakshman Telangana State Horticulture University in the month of September 2017 and was identified by the chief herbalogist. The leaves were shade dried for 5 days and ground to fine powder Figure 1.
figure 1: Showing Costus igneus leaves.

Extraction of bioactive compounds
Fine ground Costus igneus leaf powder (10g) was extracted with ethanol (150ml) using a Soxhlet’s apparatus at 60 °C for 16 hours. The extract was concentrated to 10ml at -50 ⁰C in a 0.1mm Hg vacuum using desktop Kylmakuivuri, ALPHA 1-2 LDplus, Christ model freeze dryer.
Preparation of CIBC loaded ZnNPs
90ml aqueous solution of 1.10mM Zinc nitrate was mixed with a 10mL of Costus igneus leaf extract. The samples were then centrifuged at 4000rpm for 30min to get clear supernatant. The initial concentration of the CIBC loaded ZnNP was measured using inductively coupled plasma optical emission spectrophotometer (ICP-OES, Prodigy XP, Leeman Labs, USA) and was found to be 283±0.8ppm. Then, the sample was diluted to different concentrations of 170, 100, and 80ppm and they were used to investigate the concentration-dependent antimicrobial effect of CIBC loaded ZnNPs.
Surface plasmon resonance identification
The nanoparticles were monitored by UV- visible (UV-vis) spectrum at various time intervals. The UV-Vis scannings were recorded using UV-2450, SHIMADZU Spectrophotometer. UVVis absorbance of the colloidal nanoparticles was measured at wavelengths from 200nm to 400nm against solvent blank (distilled water).
Particle size and zeta potential analysis of Zn nanoparticles
The aqueous suspension of the CIBC loaded ZnNPs was filtered through a 0.22μm syringe filter unit, and the charge, size and distribution of the nanoparticles were measured using dynamic light scattering technique (HORIBA, SZ-100).
FT-IR analysis for synthesized nanoparticles
The FT-IR spectrum was taken within the IR region of 400- 4000cm-1. The IR spectrum was recorded using ATR (attenuated total reflectance) technique. The sample was mixed with the KBr (1:200) crystal, and the spectrum was recorded in transmittance mode (Tensor 27).
Scanning electron microscopy (SEM)
The morphology of the nanoparticles was characterization by Scanning electron microscopy was performed on JEOL (JEM-1010) instrument, with an accelerating voltage of 80kvafter drying of a drop of aqueous Zn nanoparticles on the carbon-coated copper SEM grids Samples were dried and kept under vacuum in desiccators before loading them onto a specimen holder. The particle size distribution of nanoparticles was evaluated using ImageJ 1.45s software
Antimicrobial assay
Antimicrobial activity of CIBC loaded ZnNPs were determined on the basis of colony forming units (CFU) by in-vitro assays (disk diffusion). Each microbial (fungal and bacterial) isolates were cultured on media that induced prolific conidia and bacterial production. The fungal species were grown on potato dextrose agar (PDA) medium and bacterial species were grown on nutrient agar (NA) medium. A 10μl aliquot of the diluted spore suspension was spread on PDA (Becton, Dickson and Company, Sparks, MD) medium. Three PDA plates for fungi and three NA plates for bacteria per each combination of exposure CIBC loaded ZnNPs concentration were tested. The filter paper disk was dipped in different ppm and inserted on mediums, and then the agar plates were incubated at 37 °C for 2 and 4 days for fungi and bacteria, respectively. The zone size was determined by measuring the diameter of the zone in mm [4]. The positive controls were Itraconazole and Ketoconazole 30μg 5mm discs for fungi species and tetracycline, ampicillin, penicillin and CMZ 30μg 5mm dics for bacterial species. Aspergillus niger, Sclerotium sp, Rhizophus rolfziisacc and Aspergillus flavus were used to check the antifungal activity and Bacillus subtilis, Streptomyces sp, Pseudomonas fluorescence and Staphylococcus aureus were used to check the antibacterial activity of synthesized CIBC loaded ZnNPs. Distilled water was used as controls for the antimicrobial assay.
Antioxidant activity of the nanoparticles
Antioxidant activity of the CIBC loaded ZnNPs were evaluated using DPPH radical scavenging activity. A dose of 0.1ml of the colloidal CIBC loaded ZnNPs were added to 1.9ml ethanol solution of 0.1mM DPPH radical. The mixture was shaken vigorously for 1min and incubated at 30 °C in the dark for 30min. The absorbance of the sample was measured using UV-2450, Shimadzu Spectrophotometer at 517nm against ethanol blank. A negative control was taken after adding DPPH solution to 0.1ml of deionized water. The percent of DPPH discoloration of the sample was calculated according to the following equation [5].
Percent discolouration= (1-(Absorbance of the sample/ Absorbance of the control))*100
Free radical scavenging activity was expressed as an equivalent of mmol trolox standard. Linearity range of the calibration curve was 0.1 to 10μmol/ml (Correlation coefficient R2=0.9).
Results and Discussion
Surface plasmon resonance identification
The absorption spectrum was recorded for the sample in the range of 200-400nm. The spectrum showed the absorbance peak at 260nm corresponding to the characteristic localized SPR (LSPR) band of Zn nanoparticles in Figure 2.
figure 2: UV-visible spectrum of synthesized Zn nanoparticles.

Particle size and zeta potential analysis of Zn nanoparticles
Particle size and zeta potential values were measured using Nanopartica SZ-100 (HORIBA). The particle size distribution spectra for the zinc nanoparticles were recorded as diameter (nm) versus frequency (%/nm) spectra with diameter (nm) on x-axis and frequency (%/ nm) on y-axis. The zeta potential spectra for the zinc nanoparticles were recorded zeta potential verses intensity spectra with zeta potential (mV) on x-axis and intensity (au) on y-axis. Figure 3 show that the synthesized ZnNPs are stable having a -16.3 mV charge. As depicted by Figure 3 the synthesized ZnNPs were among the nanoparticle range having a mean particle size distribution of 42.3nm.
figure 3: Dynamic light scattering and particle size and zeta potential distribution of synthesized nanoparticles.

FT-IR analysis for synthesized nanoparticles
The functional groups poly alcohols, carboxylic acids, alkyl halides, alkenes, alkanes, nitrites and benzene rings were present in the ZnNPs. The peaks at 3371cm-1, 3556cm-1, 2918cm-1, 2848cm- 1, 1629cm-1, 1533cm-1, 1446cm-1, 1060cm-1, 1031cm-1, 875cm-1, and 808cm-1 in Figure 4 show the presence of these functional groups attached to nanoparticles. From the FT-IR spectra of CIBC loaded ZnNPs sample, change in wave number of the functional groups was observed due to the reduction and stabilization of metal group Zn.
figure 4:FT-IR spectra of CIBC loaded ZnNPs.

Scanning electron microscopic analysis
CIBC loaded Zinc nanoparticles used in this study is having the mean diameter of 2-20μm as shown by SEM micrographs Figure 5. The formed CIBC ZnNPs appears slightly aggregated due to the absence of strong surface protecting ligands and found to be spherical and irregular in shape. The green synthesized and characterized Zn nanoparticles were crystalline in nature with uniform size and shape so in future it may acts an alternative source for the production of metallic nanoparticles by avoiding chemical methods.While noticing the reactions at different concentration of leaf extracts shown the decreases the spectrum as the volume of the aqueous solution of leaf extract of C. igneus increases. This may be due to the presence of more reducing phytoconstituents in higher concentration of extract which results in an additional interaction between the surface capping molecules and secondary reduction process obtained through high dispersion in higher temperature.
figure 5:Scanning electron microscopic spectra of CIBC loaded ZnNPs.

Antimicrobial assay (Table 1)
Table 1: Inhibitory effect of ZnNPs (170ppm) on selected fungal strains.
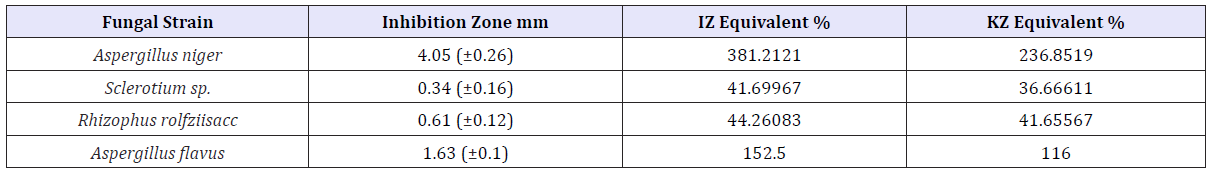
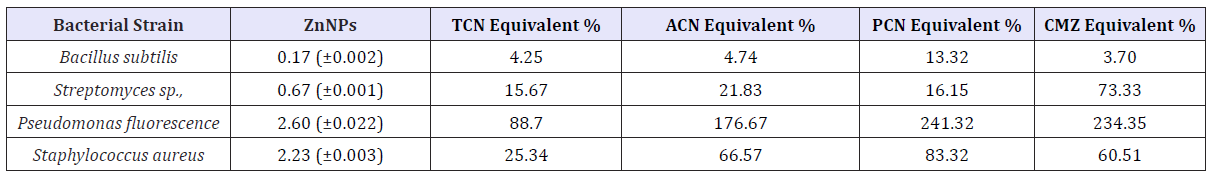
Table 2: Inhibitory effect of ZnNPs (170ppm) on selected bacterial strains.
170ppm CIBC loaded ZnNP showed significant (p<0.05) inhibitory effect on Aspergillus niger and Aspergillus flavus species and several folds inhibitory activity greater than IZ and KZ positive controls (Table 2).
A significant (p<0.05) antibacterial activity against Streptomycin sp, Pseudomonas fluorescence, and Staphylococcus aureus has been identified. ZnNPs showed more inhibitory effect on Pseudomonas fluorescence compared to other species and positive controls CAN, PCN and CMZ and equivalent antibacterial activity with TCN. During the present study, different concentrations of Zinc nanoparticles were tested to find out the best concentration that can have the most effective antibacterial property than fungi. Zinc nanoparticles synthesized from the Cinnamomum zeylanicum bark extract proved to be more potent. The antimicrobial activity is probably derived, through the electrostatic attraction between negative charged cell membrane of microorganism and positive charged nanoparticles.
It reflects that Zinc nanoparticles have an excellent antibacterial and anti-fungal effect and potential in reducing bacterial, fungal growth for practical applications, Green synthesis of zinc nanoparticles promises the up scalable and non-toxic method of production of a variety of metallic nanoparticles. Applications of Zn nanoparticles based on these findings may lead to valuable discoveries in various fields such as medical devices and antimicrobial systems (Table 3).
Table 3: Antioxidant activity of CIBC loaded ZnNPs.
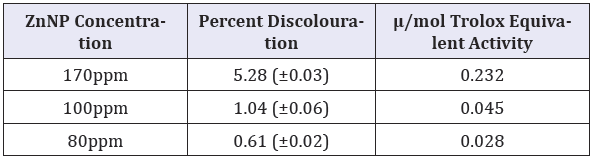
DPPH radical scavenging activity of the CIBC loaded ZnNPs were significantly higher at 170ppm (p, 0.05) and was equivalent to 0.231μ/mol Trolox equivalent DPPH radical scavenging activity.
Conclusion
The synthesized CIBC loaded ZnNP were in the range of nanoparticles and exhibited nanoproperties and bioactive properties. From the results it can be concluded that CIBC loaded ZnNPs can be a used as a stable source of antimicrobial agent and carrier of antioxidant bioactive compounds. The findings in this study may lead to the development of ZnNPs-based new antimicrobial systems for medical applications
References
- Gunalan S, Sivaraj R, Rajendran V (2012) Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Progress in Natural Science: Materials International 22(6): 693-700.
- Singh RP, Shukla VK, Yadav RS, Sharma PK, Singh PK, et al. (2011) Biological approach of zinc oxide nanoparticles formation and its characterization. Adv Mater Lett 2(4): 313-317.
- Thiruchenduran S, Maheswari KU, Suneetha J, Prasad TNVKV, Rajeswari B (2016) Screening for plant secondary metabolites in selected indigenous herbal plants. Imperial Journal of Interdisciplinary Research 2(4): 1103- 1106.
- Yamaç M, and Bilgili F (2006) Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushroom isolates. Pharmaceutical biology 44(9): 660-667.
- Xu BJ, Chang SKC (2007) A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J food sci 72(2): S159-S166.
© 2018 Thiruchenduran S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






