- Submissions

Full Text
Research in Medical & Engineering Sciences
Stimulus Activated Shape Switching in Shape Memory Materials with Tailor Able Start/Finish Temperature/ Time
Tao Xi Wang and Wei Min Huang*
Nanyang Technological University, Singapore
*Corresponding author: ANanotechnology laboratory, Institute of Frontier Technology, Regional Agricultural Research Station, Acharya N G Ranga Agricultural University, Tirupati-517502, AP, India
Submission: September 12, 2017; Published: September 19, 2017

ISSN : 2576-8816Volume1 Issue3
Editorial
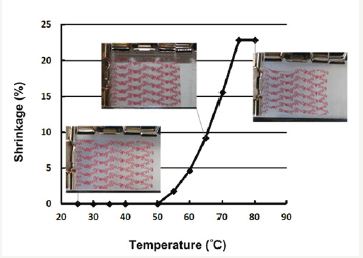
The shape memory effect (SME) refers to the phenomenon of shape recovery in a severely pre-deformed material, but only when a right stimulus is applied. The materials with the SME are called shape memory material (SMM), and typical stimuli include heat, chemical (including water/moisture), light, etc [1]. Right now, the family of SMM has expended significantly, and covers a range of different types of materials, including alloy, polymer and ceramic etc [2-5]. In general, the SME process includes two steps [6]. The first step is to program the piece of SMM into a temporary shape (known as the programming process); and the second step is to apply the right stimulus for recovery (called the recovery process) with or without constraint applied. However, the start and finish of recovery is normally dependent on the material itself. For instance, in Figure 1, a piece of heat shrink polymeric film starts to shrink upon heating to its glass transition start temperature, which is about 50 oC. Hence, traditionally, in order to meet the recovery temperature requirement of a particular application, we have to search for a polymeric material with the required glass transition start temperature or melting start temperature. Although most of polymeric materials are heat/chemo-responsive SMM, it is still
Figure 1:Shrinkage (in %) of a piece of typical one-direction (vertical direction in this particular test) heat shrink label with an as-received thickness of 50cm. (Reproduced from [6] with permission).
a tedious process to find the right material, since there are many other requirements, such as biodegradability, shape recovery ratio etc, in the application as well.
Keywords
Shape memory material; Stimulus; Switching time; Switching temperature
Introduction
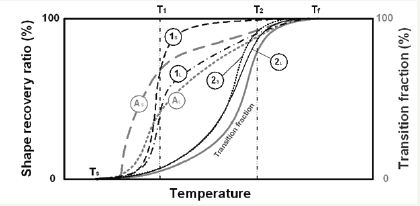
As revealed in [7], the recovery start temperature of a polymeric material is actually tailor able within its glass transition or melting temperature range. That is to say, provided that the programming temperature is within the glass transition or melting temperature range (between Ts and Tf in Figure 2), the recovery temperature is around the programming temperature in the final heating process [refer to the line of (1s) in Figure 2. However, as pointed out in [8], this is only applicable to small programming strain. If the applied programming strain is relatively larger (which is material dependent), the actual recovery start and finish temperatures are within a wider range, and in particular the recovery finish shifts to a higher temperature range.
Figure 2:Shape recovery ratio and transition fraction against heating temperature curves. AS and AL: recovery ratio upon heating to the programming temperature. Subscripts S and L denote small strain and large strain, respectively. (Reproduced from [8] with permission from Springer).
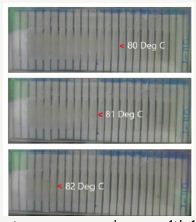
Utilizing this feature, we can tailor the shape recovery temperature of a polymeric material. Figure 3 presents an example for temperature sensing application, in which a piece of polystyrene plate (2.5mm thick) is pre-impressed using a carefully designed, customized mould at one temperature, which is within the glass transition range of this polystyrene. Upon subsequently gradual heating from 8 oC to 82 oC the dark lines in the middle appear in a step by step manner from the right towards the left. Hence, after calibration, we may monitor the actual highest temperature in a heating process. Such a kind of temperature sensor requires no battery for operation and the result can be directly observed by naked eyes [9].
Figure 3:One-step programming, multiple-step recovery upon gradual heating in polystyrene. (Reproduced from [9]).
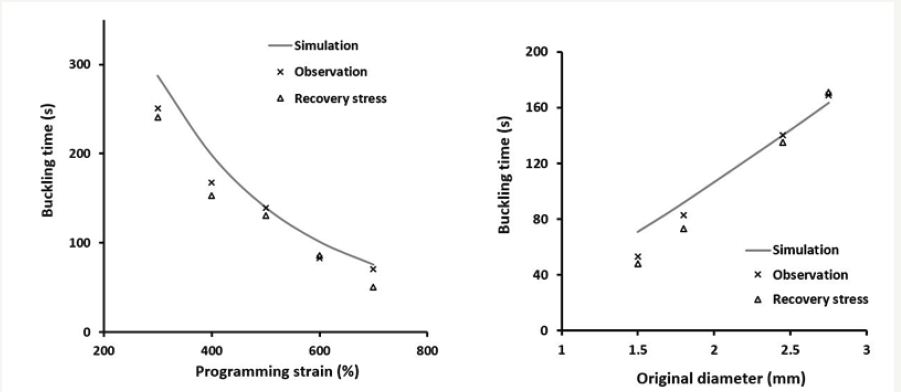
In addition to tailor the start and finish temperatures in heating induced recovery as mentioned above, we may pre-determine the start and finish time of a chemo-responsive shape recovery process as well. As shown in Figure 4 (top), a piece of photo-cross-linked poly (ethylene glycol) (PEG) swells gradually after being immersed in room temperature water (about 22 oC). However, a piece of wire made of the same PEG, if pre-stretched at high temperatures and then immersed in room temperature water, buckling occurs after about 80 s to 88 s (Figure 4, bottom). The observed buckling phenomenon is due to water-induced SME. For a particular material, the actual buckling time is controlled by the amount of programming strain and original thickness of the PEG wire, and can be pre-determined as shown in Figure 5a & 5b. Since the PEG used here is biodegradable, such a buckling phenomenon has been proposed for biodegradable occlusion for liver cancer treatment [10,11]. The SME with tailor able start and finish temperature/time provides great flexibility to control shape switching in a pre-determined fashion. It has great potential in many biomedical applications, such as in biomedical devices in, for instance, sequentially controlled folding/unfolding for minimally invasive surgery.
Figure 4:Swelling induced shape change (top) and buckling induced by the SME (bottom) (Reproduced from [11] with permission).

Figure 5:Buckling time (a) as a function of programming strain (original diameter: 2.45mm) and (b) as a function of original diameter at a fixed programming strain of 500%. (Reproduced from [11] with permission).
References
- Huang WM, Ding Z, Wang CC, Wei J, Zhao Y, et al. (2010) Shape memory materials. Mater Today 13(7-8): 54-61.
- Otsuka K, Wayman CM (1998) Shape memory materials. Cambridge University Press, UK.
- Lendlein A, Kelch S (2002) Shape-memory polymers. Angewandte Chemie-International Edition 41: 2034-2057.
- Swain MV (1986) Shape memory behaviour in partially stabilized zirconia ceramics. Nature 322: 234-236.
- Zhou Y, Huang WM, Zhao Y, Ding Z, Li Y, et al. (2016) Memory phenomenon in a lanthanum based bulk metallic glass. Journal of Alloys and Compounds 672: 131-136..
- Wu XL, Huang WM, Lu HB, Wang CC, Cui HP, et al. (2017) Characterization of polymeric shape memory materials. Journal of Polymer Engineering 37: 1-20.
- Sun L, Huang WM (2010) Mechanisms of the multi-shape memory effect and temperature memory effect in shape memory polymers. Soft Matter 6(18): 4403-4406.
- Huang WM, Zhao Y, Wang CC, Ding Z, Purnawali H, et al. (2012) Thermo/ chemo-responsive shape memory effect in polymers: a sketch of working mechanisms, fundamentals and optimization. Journal of Polymer Research 19: 9952.
- Yang WG, Lu HB, Huang WM, Qi HJ, Wu XL, et al. (2014) Advanced shape memory technology to reshape product design, manufacturing and recycling. Polymers 6(8): 2287-2308.
- Wong YS, Vijay SA, Zhuang DK, Liu H, Birch WR, et al. (2016) Bioabsorbable radiopaque water-responsive shape memory embolization plug for temporary vascular occlusion. Biomaterials 102: 98-106.
- Salvekar AV, Huang WM, Xiao R, Wong YS, Venkatraman SS, et al. (2017) Water-responsive shape recovery induced buckling in biodegradable photo-cross-linked poly (ethylene glycol)(PEG) hydrogel. Acc Chem Res 50: 141-150.
© 2017 Tao Xi Wang, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






