- Submissions

Full Text
Research & Development in Material Science
Phosphate & Nitrate Removal from Agricultural Runoff by Chitosan-Graphite Composite
Rajendra S Dongre*
Department of Chemistry, RTM Nagpur University, India
*Corresponding author: Rajendra S Dongre, Department of Chemistry, RTM Nagpur University, Nagpur-440033, India
Submission: April 25, 2018;Published: May 15, 2018

ISSN: 2576-8840
Volume6 Issue3
Abstract
Agriculture hugely used varied synthetic fertilizer/pesticide as nutrients and during periods of rain, these unused nutrients percolate from fertilized soils into the water-streams/rivers. Oozing excessive nutrients, mostly phosphorus and nitrogen causes water pollution and eutrophication, thus detrimental to ecosystem. This research synthesized benign bio-composite from naturally abundant chitosan by doping with graphite and SiO2 to remove nitrate/phosphates contamination from agricultural runoff/drainage percolating through water in batch adsorption mode. At lower concentrations (10-25mg/L), while at higher concentrations (up to 100mg/L), removal efficiency decreased, highest removal (70%) at 25-mg/L nitrate, while at higher concentrations (50 and 100mg/L), nitrate removal 36.7 and 42.9%,. 50% mix showed best performance in terms of both rates and percentage of removal for most of nitrate investigated indicating that a higher amount of in mixes improves adsorption capacity. Both Langmuir and Freundlich isotherm models applied to experimental data and Langmuir described better nitrate removal process. Accordingly, graphite doped chitosan composite is benign alternative to the commercial adsorbents to reduce the cost of water treatment with enhanced nitrate/phosphate removal capacity. The saturated adsorbents after adsorption of nitrate was eluted with 0.1N NaOH solutions for desorption and resultant effluents were collected at regular time intervals and analyzed for residual nitrate ion chromatogram.
Keywords: Nitrate; Phosphate; Nutrient removal; Benign; Bio-composite; Chitosan; Water
Introduction
Nitrate and phosphate are vital nutrients for growth of plants, hence huge quantity added in agricultural fields and unused nutrients percolate via water, thus responsible for serious environmental problem called eutrophication. Eutrophication is slow-aging of water body, due to exceeds seasonal loading of nutrients which promotes excessive growth of algae and as algae dies/decomposes to high levels of organic matter which depletes water-oxygen, causing subsequent death of fish/aquatic species/ ecology besides endanger to human [1]. About 50% crop yields by commercial fertilizer which affects global populations as 2.5% land of terrestrial ecosystems suffered leaching load of nitrate/ phosphorous @ 30kg/ha year [2]. Rather phosphorus level >0.025ppm percolating from agricultural soils has also imparting the eutrophication [3]. Thus, nitrate/phosphate contaminations in water become serious environmental issue worldwide [4]. Nitrates/phosphate contaminations are due to agricultural runoffs besides untreated municipal disposal, domestic and industrial wastes. Amid, phosphorus found in rocks/mineral deposits gets gradually release the phosphorus as phosphate during weathering. Phosphates are not toxic as such unless present in very high levels that arise digestive problems. Phosphates 0.1ppm level is acceptable to avoid accelerated eutrophication, but level >0.1ppm accelerated consequent problems. The criteria for total phosphorus as recommended by US EPA [5] stated that no more than 0.1ppm for streams not enter into reservoirs and no more than 0.05ppm for streams discharging into reservoirs besides no more than 0.025mg/L for reservoirs. Nitrate limit of 15ppm in potable water for discharge is permissible while USEPA established a limit of 10ppm [6]. Excessive water nitrate cause methemoglobinemiae illness (ingestion in human digestive system alters oxyhemoglobin to metheglobin, which cannot carry oxygen, so tissues deprived of oxygen and arise blue coloration of mucous membranes) and digestive and respiratory disturbances [1-3]. Excess nitrates are responsible for stomach cancer in adults and symptoms of nitrate poisoning includes breath shortening, rapid heartbeat, frequent urination, body collapse, in series cases coma and death may result within a few hours [7]. Phosphates are not toxic unless present in very high levels but high phosphate levels causes digestive problems although nutrients are found moderately in natural water systems, but high level contamination mainly reliant due to huge fertilizers consumption [6,7].
Nitrate and phosphate owns high stability and solubility besides less precipitation tendency and so, difficult to remove by conventional water treatment technologies [8]. Certain physicochemical/biological methodologies like adsorption, ion exchange, reverse osmosis, and a combination of several processes developed to remove nitrate and phosphate from water. Amid, adsorption is most attractive due to its ease of operation, simplicity of design and economically more feasible especially when low-cost/ bio-adsorbent is used as adsorbents [9]. Thus varied adsorbents viz; carbon materials, agricultural wastes, ion exchange resins and synthetic organic/inorganic compounds were used to remove nitrate and phosphate from water. Nevertheless, adsorption capacities of these materials are very less, so it’s essential to find more effective/cheaper adsorbents with higher adsorption capacity. Commercial activated carbon owns low capacity to retain phosphate and adsorption capacity becomes higher with increasing phosphate concentration while presence of nitrate has no influence in phosphate adsorption. Thus, their removal gets promising concern by techniques so as to minimize post-treatment operation and maintenance costs enable bio-waste usages like chitosan sea food-industrial residues/waste has been desired. In this research chitosan is preferred due to availability of multiple adsorption sites in yielded bio-composites which gets utilized for nitrate/phosphate sorptions.
Table 1: Characteristics of FCDGNC Bio-composite, pure chitosan and pure graphite.
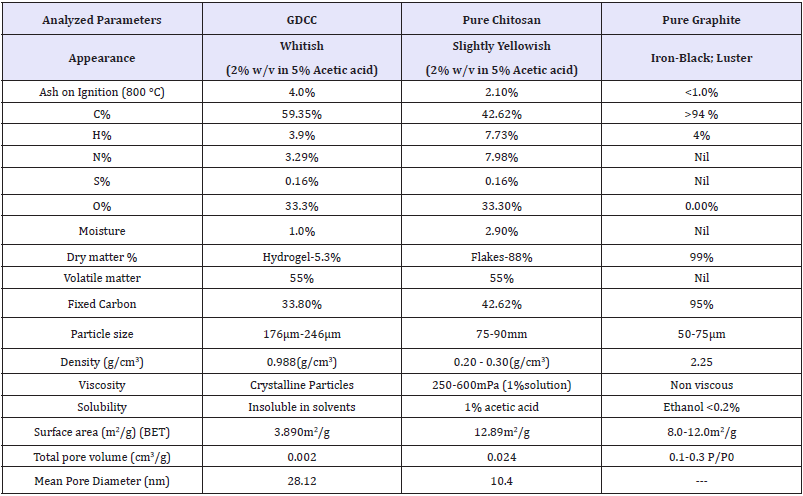
Elementary analysis (viz; C/H/N/S/O, %ash, %moisture, surface parameters): The elementary analysis shown in Table 1, Ash content calculated by Eqn (1), % moisture by gravimetry and water mass/weight difference of wet-dried sample and calculated by Eqn (2):
% Ash = [Weight of residue (g ) / Sample weight (g )] ×1(1)
% Moisture Content = [Wet weight (g ) – Dry Weight (g )] /Wet weight (g ) ×10 (2)
Determination of reaction equilibrium time: Time needed to attain adsorption equilibrium was determined after optimized pH for the sorption. Nitrates/phosphates sorption was conducted in 100mL beakers placed on shakers. Samples (50mL) were analyses after 0,1,5,10,15,20,30,40,50,60,90,120,150,180,210 and 240min. The parameters of analyses of pH value effect on the effectiveness of nitrate sorption on chitosan sorbents as shown in Table 2 & 3.
Table 2: Varied parameters effect on effectiveness of nitrate sorption on adsorbent.
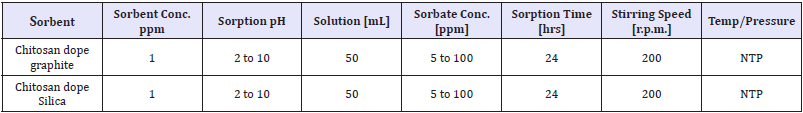
Table 3: Varied parameters effect on effectiveness of phosphate sorption on adsorbent.
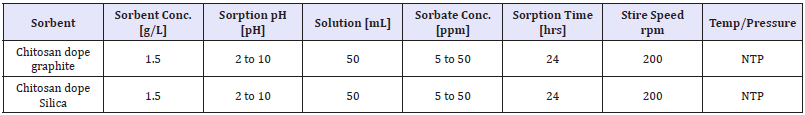
Eluate tests and batch experiments: Eluate tests were performed in distilled water over 24h, using the same liquid (mL) to solid (g) ratio applied for the batch tests (10:1) to determine leaching capacity. Parameters measured after 24h were pH, conductivity (C), total phosphate and nitrate concentration (NO3¯). All these analyses were conducted in duplicate for accuracy and in separate vials in order to avoid contamination. The batch experimental studies were conducted in order to determine nitrate/phosphate removal capacity of the synthesized adsorbents. Tests were carried out in duplicate, under uncontrolled pH condition in bottles with a liquid to solid ratio of 10:1. A total liquid volume of 50mL was used for all batch tests. The nitrate removal efficiency was tested using 5,10,25,50 and 100ppm synthetic solutions of potassium nitrate (KNO3¯) and prepared distilled water. The samples were collected during the adsorption experiments until complete removal was achieved or a plateau obtained.
Adsorption and kinetic experiments: Adsorption of nitrate/ phosphate onto adsorbents studied in batch experiments, as 1 to 1.5g adsorbent with 50mL nitrate/phosphate adsorbate taken in 100ml viols with different concentrations and kept on mechanical shaker at 200rpm, given enough contact time so as to reach equilibrium. The initial nitrate concentration was in the range from 5 to 100ppm while that of phosphate, 5 to 50ppm. Dynamic adsorption analysis was also carried out and adsorption kinetic rates of nitrate and phosphate were measured in terms of time in an agitated reactor with a water jacket to maintain a constant adsorption temperature. After the adsorption study, the collected samples were filtered through 0.45μm glass syringe filters of Whatman-Cat No. 6786-2504 and residual nitrate/phosphate in filtrates was determined with average values. All the batch adsorption experiments were carried out in duplicate and average values are reported. The percentage removal was calculated using equation (3)
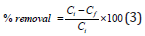
Adsorption capacities were calculated using equation (4) and (5) respectively.
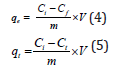
Where qe and qt were the amount of adsorbate which gets adsorbed at equilibrium and at time t respectively in (mg/g); Ci and Cf were the initial and final adsorbate concentration in (mg/L). Ct=residual adsorbate concentration at time t in (mg/L); V=volume of solution (L) i.e., is volume of nitrate and phosphate solutions, and m=mass of adsorbent (g/L).
Adsorption of nitrate and phosphate: The correlation of experimental adsorption results with the adsorption isotherms helps us to understand the adsorption process more theoretically. In this study, the following adsorption isotherm models were used to correlate the experimental results Eqn 6 and 7 below [10,11].
Langmuir; Qe = Qmaxb Ce1+ bCe (6)
Freundlich; Qe = KCe1/ n (7)
Table 4: Adsorption isotherm parameters for nitrate on chitosan doped adsorbents.
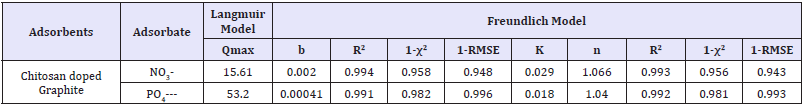
Ce is the equilibrium concentration of the adsorbate/nitrate/ phosphate in aqueous solution ppm (mg/L), Q max is the maximum adsorption capacity (mg/g), b is Langmuir constant relates binding sites affinity (L/mg), K and n are Freundlich constant relates adsorption capacity (L/g) and adsorption intensity/heterogeneity factor=Langmuir isotherm is applicable for monolayer adsorption in homogeneous manner on adsorbent surfaces. Amid, best fitting for the adsorption were decided by study of correlation coefficient (R2), residual root mean square error (RMSE), and chi-squared test (χ2) values. The largest value of R2 (te smallest values of RMSE and χ2) indicated better fitting and similarity of model with adsoprtion data due to their definitions as follows. The correlation coefficients, R2 are in range of 0.979-0.994 and 0.969-0.994 for Langmuir and Freundlich isotherm models, respectively. Based on the obtained values of R2, 1-RMSE, and 1-χ2, Langmuir equation-3 looks more accurate than Freundlich as mentioned in Table 4: so as to describe adsorption equilibriums with represented fitting ability. However, the unreasonably large values of qm and small values of b, in case of Langmuir isothermal parameters imply that it’s not proper in fitting equilibrium (as some data almost linear/unfavourable). In these cases, linear isotherm/Freundlich seems proper to simulate adsorption process. The adsorption of nitrate/phosphate also decreases slightly with enhancing temperature gets linear/slightly unfavourable implied physical adsorption predominates and removal is difficult.
Scanning electron micrographic (SEM): Scanning electron micrographic images of adsorbent Figure 1 showed porous morphology with different pore size/shapes with flaky, smooth, shiny appearance and some voids/cavities, where graphite entered chitosan skeleton giving high surface area required for adsorption.
Figure 1: SEM images of GDCC at (a) 1500X (b) 4000X (c) 5000X.
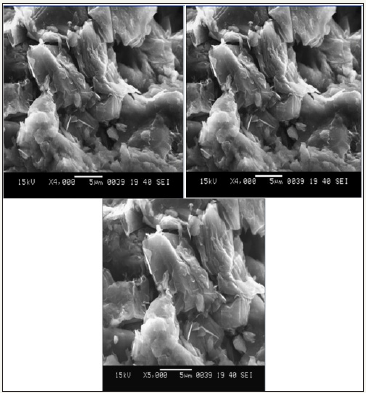
Figure 2: Powder XRD pattern of
2a: Pure chitosan
2b: Graphite
2c: Graphite doped chitosan.
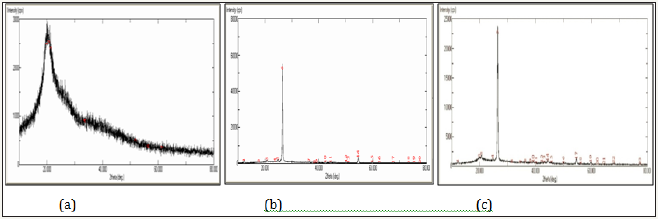
XRD: XRD pattern of pure chitosan, pure graphite and graphite doped chitosan is illustrated in Figure 2a-2c respectively. X ray diffraction pattern of chitosan exhibited broad diffraction peak at 2θ=20° with d- spacing of 4.2Å is characteristics of semi crystalline chitosan [12]. A very small peak appeared at 2θ=20.74° reported in chitosan and peak at 2θ=20.2° that matches well with the literature [13]. The peaks are broadened due to the amorphous nature of chitosan skeleton. There is no impurity peaks observed in the XRD images of pure chitosan. The diffraction peak appeared at 2θ=26.5° which indicated d- spacing of about 3.35Å is a characteristic of graphite peak [14-16]. The XRD pattern of the graphite doped chitosan indicated the formation of single phase composite and the peaks were obtained at 2θ value 26.5. The broad peak at around 2θ=20° which was due to the chitosan decreased in intensity after doping with graphite which confirms that graphite is doped on the surface of chitosan. A predominant peak of graphite along with small peak of chitosan appeared in doped adsorbent showed graphite incorporation in chiotsan matrix successful and effectively to provide support adsorbent active sites.
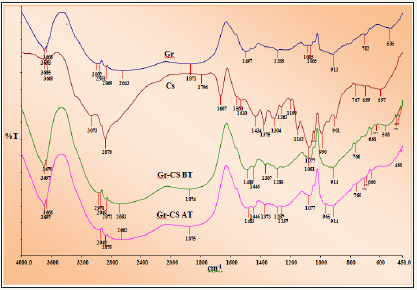
FTIR analysis: FTIR spectra of chitosan powder, graphite powder and chitosan/graphite blend before and after adsorptions of adsorbate are presented in Figure 3. Pure chitosan showed characteristics absorption band namely 3695cm-1 and 3073cm-1 for stretching vibration of -OH group. Absorption band at 2876cm- 1 in range of 2800-2950cm-1 are assigned to the hydroxyl groups present in chitosan. 1667cm-1 for carbonyl C=O stretch in amide and 1152cm-1 for bridge-O-stretching. Bending vibrations of methylene/ methyl groups found peaks at 1375cm-1 and 1434cm-1 respectively. The absorption peak at 1262cm-1 attributed for C-O-H stretching. The broad peak at 1077cm-1 ascribed to C-O stretching vibration of the ring C-O-H, C-O-C and CH2OH [12,17]. The absorption peak of chitosan at 1667cm-1 for C=O stretch in amide was disappeared on graphite doping. Similarly absorption band of chitosan at 1375cm-1 for C-H stretch was remarkably shifted to lower wave number of 1367cm-1. These two polymers i.e., chitosan and graphite are mixed and changes characteristic FTIR peaks due to reflection of physical blends/ interactions. These observations indicate the existence of good miscibility and doping between chitosan and graphite. The spectral analysis of graphite doped chitosan before and after adsorption of adsorbate showed that the peaks either decreases in intensity or disappear might involve in these anionic adsorption.
Figure 3: FTIR spectrum of (a) pure graphite, (b) pure chitosan, (c) Graphite doped chitosan composite before adsorption (d) Graphite doped chitosan after adsorption.
Adsorption isotherm: The relationship between the amount of substance adsorbed per unit mass of adsorbent at constant temperature and its concentration in the equilibrium solution is called as adsorption isotherm. Equilibrium adsorption isotherms are vital to determine sorption capacity of phosphate/nitrate onto graphite doped chitosan. The experimental data were fitted into the Langmuir and Freundlich isotherm models. The kinetic parameters of phosphate and nitrate adsorption onto chitosan doped adsorbents were determined from the pseudo-first order and pseudo-second order models, as provided in Table 5. The reaction rate constants and chitosan sorption capacities for nitrate/ phosphate were influenced by their initial concentrations. In the mixture of nitrate/phosphate, regardless of concentration, chitosan sorbent showed highest sorption capacity for phosphates.
Table 5: Kinetic of nitrate/phosphate adsorption from pseudo-1st and 2nd order model
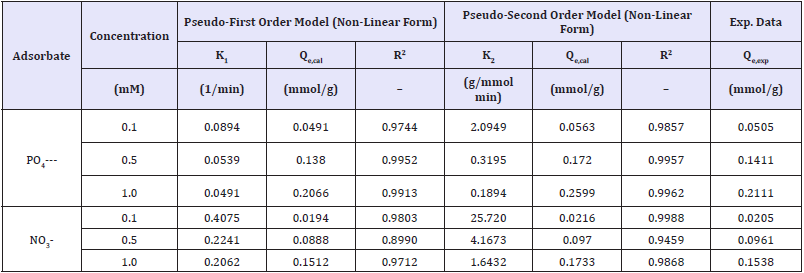
Figure 4:
4a: Intra-particle diffusion model for phosphate
4b: Intra-particle diffusion model for nitrate.
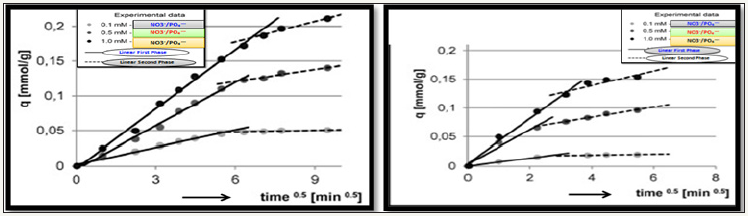
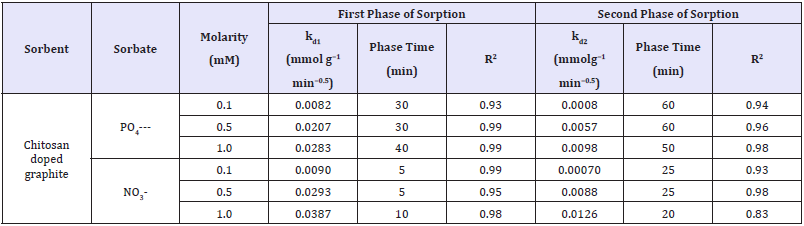
The intraparticle diffusion model found well fitted to experimental data indicated sorptions of nitrate/phosphate proceeds in two phases presumably, first adsorption of nitrate/phosphate anions by active sorbent’s surface, whereas second included slow absorption of nitrate/phosphate as shown in Figure 4a & 4b. During both phases, nitrate/phosphate anions get penetrated into the composite structure of chitosan and exploits sorption underneath sorbent’s surface.
Nitrate/phaophate sorption effectiveness found higher in the first phase of adsorption, compared to the second phase; however, first phase was much shorter. In the initial 10 minutes of adsorption the most intensive was the sorption of nitrates, which was indicated by high values of kd1 constant. A higher rate of nitrate was most probably due to the lower molecular mass than phosphate and their greater capability to penetrate the composite structure adsorbent. Then sorption of nitrates evolved into second phase with significant lower sorption after 30-40min and then nitrates sorption ended, whereas phosphate sorption already evolved into the second phase and lasts for some more time 50-60min. Owing to such prolonged sorption, phosphate anions gets more adsorbed onto as compared to nitrates as shown in Table 6.
Table 6: Adsorbate diffusion rate constants from intra-particle diffusion model.
Maximum adsorption capacity
Figure 5:Isotherms of nutrients sorption: A phosphate/nitrates onto chitosan composits (from quimolar mixture) at NTP.
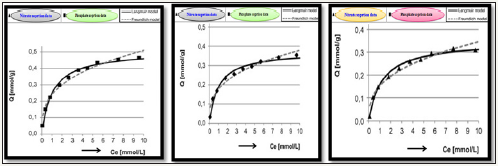
The adsorption isotherms of phosphate/nitrate sorption onto a chitosan composite sorbent are plotted in Figure 5. Data obtained were described using heterogeneous Langmuir’s model and Freundlich model. In each case, best fit to experimental data shown for Langmuir model.
In the Langmuir’s theory, the binding of sorbate molecules with active adsorbent sites may be chemisorptions/physical in character (e.g. electrostatic interactions, hydrogen bonds). The sorbed adsorbates don’t interact with each other and do not form a multilayer. They may, however, change the active centers and be substituted by sorbate from the solution. The mass of the sorbed substance is influenced by the maximum sorption capacity of a sorbent monolayer (Qmax), and by sorbate affinity to sorbent (Kc). The analysis of adsorption isotherm shape and a relatively low value of Kc constant suggested nitrate/phosphate sorption capacity as largely depended on their concentration in solution (Figure 5). The sorption capacity of 1g of adsorbent reached 1.2mmol of all nitrate/phosphate. Amid the used adsorbents for nitrate/phosphate sorption, chitosan showed the highest affinity to phosphates reached 0.51mmol PO4−/g, and 0.36mmol NO3−/g, respectively. Thus the phosphate/nitrate affinity at sorption ‘sites in form of hydrogel beads is ordered as follows: PO4− >NO3−. The isothermal adsorption constants and sorption capacities were determined from Langmuir’s and Freundlich model as summarized in Figure 5, whereas comparison of the effectiveness of nutrients sorption on various sorbents is presented in Table 7 & 8.
Table 7: Langmuir’s and from Freundlich Constants determined at same conditions.
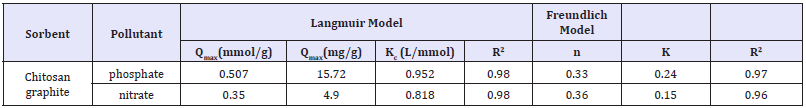
Table 8: Comparative sorption capacity of varied adsorbents for phosphates.
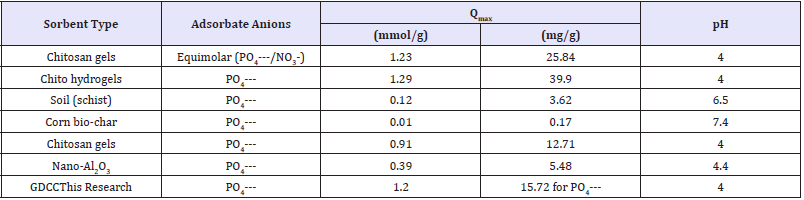
Table 9: Comparative adsorption capacity for nitrate removal from water.
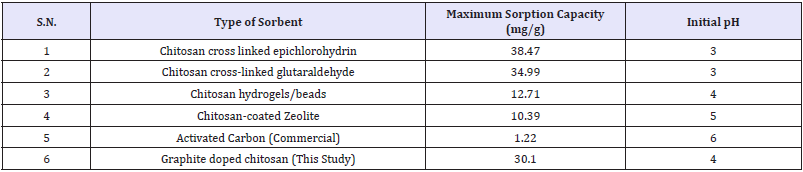
Among the literature reported and synthesized chitosan sorbents, comparative highest affinity to nitrate/phosphate is found for graphite doped chitosan sorbents [11-17] as shown in Table 9. The relatively sorption capacity of 1g of adsorbent for 1.29mmol phosphate and 0.9mmol nitrate was similar, and pointed similar adsorption via binding and to competition between anions for adsorbent active sites. The graphite doped chitosan composites were successfully applied for nitrate/phosphate sorption from aqueous solutions. The effectiveness of NO3- sorption onto chitosan sorbents increases along with a decreasing pH and lowest pH=4/ acidic conditions enhances sorption capability. Nitrates sorption at lower pH, i.e. pH 2-3, requires the use of cross-linked chitosan which is resistant to dissolution at low pH may significantly increase sorption capability of adsorbent higher compared to unmodified/ pure chitosan. An advantage of doping onto chitosan skeleton enhanced its high sorption effectiveness in a wide pH range (pH 2-6). Kinetics of nitrates sorption onto doped chitosan is best described with the pseudo-second order model and intraparticle diffusion model points to three stages of nitrate sorption [11]. The equilibrium time of the sorption process for doepd chitosan composites reaches 180min, but most nitrates sorbed within first 30min of process.
Effect of pH on effectiveness of nitrate/phosphate sorption: The adsorption of analyzed nutrients (nitrate/phosphate) found to be influenced by solution pH. In a pH value range from 4 to 11, the effectiveness of (nitrate/phosphate) sorption onto chitosan in the form of hydrogel composites decreased with an increasing initial pH of solution Figure 6, similar tend observed for nitrate/ phosphate sorption onto polymer-chitosan composites [12-14].
Figure 6: Effect of pH on effectiveness of nitrate/phosphate sorption onto doped chitosan.
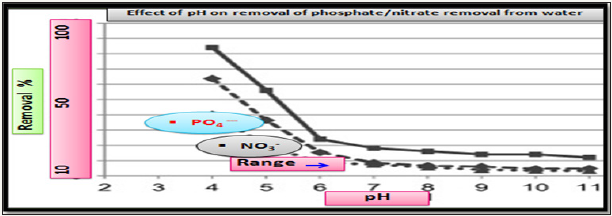
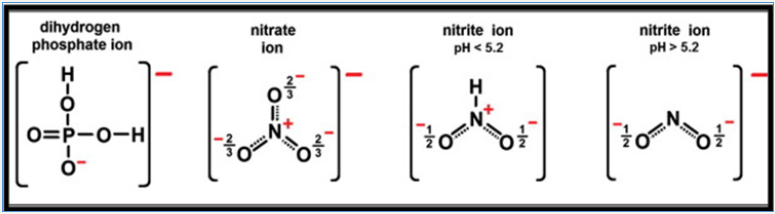
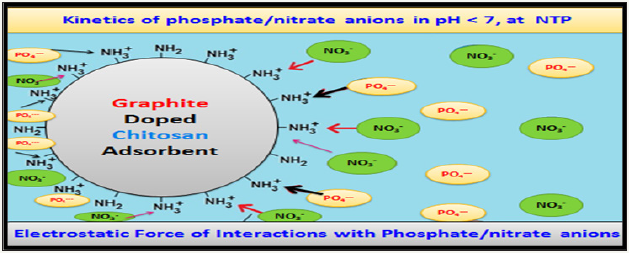
The nitrate/phosphate adsorption capacity by chitosan composite was resulted due to the electrostatic attraction between protonated amine of chitosan with anionic adsorbent sites. As number of protonated amino groups increases (at low pH) then the sorption of nitrate/phosphate increases as solution pH decreases. For this reason, the effectiveness of anionic nitrate/phosphate sorption was the highest in acidic solutions at pH 4 and above initial pH >4, the sorption of nitrate/phosphate onto adsorbent surfaces was ineffective and the lowest sorption was determined in a pH 8 to 11 range. But in alkaline pH, the chitosan surface attains a negative charge, and gets electrostatic repulsion with anionic repulsion to prevent their sorption. The very low repulsive sorptions at high pH are also due to competition between and OH− ions nitrate/ phosphate anions at the sorption centers of adsorbents. At the initial pH <4 the electrostatic repulsion between chitosan skeleton found so strong that the chitosan sorbent gets dissolved and lost its adsorption ability so, adsorption between initial pH 2-3 were excluded in Figure 4. A lower effectiveness of nitrates sorption onto chitosan are due to weaker electrostatic interactions with sorbent’s surface. In the pH range from 4.0 to 5.0, most of nitrates possessed the total neutral charge to suppress the electrostatic interactions with protonated –NH3+ of graphite doped chitosan. Thus negative charge of nitrates found distributed among a few oxygen and poorer electrostatic interactions of nitrates with active sites of adsorbents gets induced by dispersion besides impaired sorptions. And phosphate ion exists as dihydrogen phosphate at acidic conditions and charge gets concentrated which increases interactions with protonated amines as shown in Figure 7.
Figure 7: Structures of nitrate/phosphate anions in a pH range from 2 to 6.5.
Chitosan based adsorbent own a great influence on pH changes during sorption and with pH 4 to 9 range, whit decreased sorption and pH 7 to 8 regardless of ionic strength of solution. This is anticipated in the wastewater treatment processes because the solution neutralizes itself throughout the sorption and adjustment of its pH after treatment is unnecessary.
Results and Discussion
Effect of graphite doping
The effect of graphite doping on chitosan for removal of nitrate/ phosphate were studied from 5% to 30% w/w. It was observed that 20% of graphite doping is optimum and higher graphite doping induced leaching from composite skeleton besides the permissible limit in water gets achieved with mere 20% graphite loading.
Effect of adsorbent/composite dose
Adsorbent dose affected adsorption at fixed initial nitrate/ phosphate concentration as shown in Figure 8. As absorbent dose enhanced, the nitrate/phosphate removal found increased but, loading capacity found gradually decreased. The maximum nitrate/ phosphate removal capacity was 3.5mg/g for 2.5g/l of dose as shown in Figure 8. Initially, higher nitrate/phosphate adsorbed per unit mass of adsorbent (due to more adsorbate/adsorbent ratio) which continued to increase up to dose of 10g/L adsorbent. Further increase in adsorbent doses not showed appreciable progress in nitrate/phosphate removal relatively negligible sorption.
Figure 8: Adsorption Kinetics of phosphate/nitrate anions onto graphite doepd chitosan.
Effects of pH
Effect of pH value on the effectiveness of nitrate sorption onto chitosan adsorbents was found to increase along with a decreasing initial pH and highest at an optimal pH 4, the removal rate reached 70%, 81% and 95% for initial nitrate concentrations of 10, 50 and 100mg nitrate. At optimal pH, the effectiveness of phosphate sorption reached 48%, 40% and 34%. The increased effectiveness of nitrates sorption at low pH may be caused by chitosan-amine group’s protonation and electrostatic forces between positivelycharged sorbent and negatively-charged nitrate/phosphate anions significantly enhanced the adsorption process. Such type of impact at low pH conditions for nitrates adsorption also investigated with activated carbon [10], sepiolite [11] and aluminium oxide [12]. But at pH >7 (alkaline medium) OH- ions competes with nitrate/ phosphate and significantly impaired ad sorption. Amid analysed adsorbents, pure chitosan displayed the lowest adsorption capability against nitrate/phosphate. The sorption of nitrate/ phosphate was significantly more effective upon the use of doped chitosan composites; however, this processes required pH value adjustment to pH 3. The best sorbent of nitrate/phosphate turned out to be graphite doped chitosan shows high sorption capability in a wide range of pH values (pH 2-6).
Effect of agitation speed
In adsorptions agitation speed is vitally affecting the external boundary film and the distribution of nitrate/phosphate in the bulk solution. Hence, agitation speed was varied in range of 50-300rpm for adsorption experiments. Initially, adsorbate adsorption was lower at 50, 100 and 150, but enhanced at 180 and remains steady >200rpm (so kept optimal speed) attributed to little boundary layer resistance besides high mobility.
Adsorption-desorption cycle/Regeneration of adsorbent
Regeneration/desorption for adsorbed chiotsan composites were performed at pH=8 after every cycle of adsorption. Initial electrostatic binding of nitrate/phosphate onto amino/hydroxyl gets weakened drastically in extreme basic pH>8 due to competition with OH. Literature, supports decreased nitrate/phosphate removal at pH>7 due to inability of proactive sites of composite. It’s known that at basic conditions (pH>7) chemisorptions decreases, due to deprotonation of chitosan surface. The adsorbent was regenerated after its use in every adsorption mode and adsorbents was reused in four cycles of adsorption with small reduction in removal or decreased nitrate/phosphate capacity.
The adsorbent is economic if gets regenerated as it reduces environmental impacts of solid waste disposal. The phosphate/ nitrate desorption from graphite doped chitosan composite and its regeneration was done at pH 10 using 0.1N alkali solution. About 90% of adsorbed pollutant was desorbed; indicated it’s a reversible phenomenon. But, residual fraction of adsorbed pollutants which would not get desorbed showed tight bonding at cationic sites of adsorbents. The regenerated adsorbent was reused in other experiments with somewhat retained of original capacity. This little change in removal capacity may be due to residual/un-desorbed pollutants previously available onto active surfaces of adsorbent. Further adsorption–desorption optimization cycle needs to improve its regeneration capacity.
Adsorption mechanism
The proponents of nitrate/phosphate adsorption argued its economical efficiency occurred in following phases and depicted in Figure 8:
1. Adsorbate diffuses to surface from bulk across boundary layer via external mass transfer;
2. Adsorption of dsorbate on to particle surfaces of adsorbent;
3. Sorbed adsorbate exchange with structural elements inside adsorbent and transferred to internal surfaces for porous via intra particle diffusion [15].
Preparation scheme for graphite doped chitosan composites
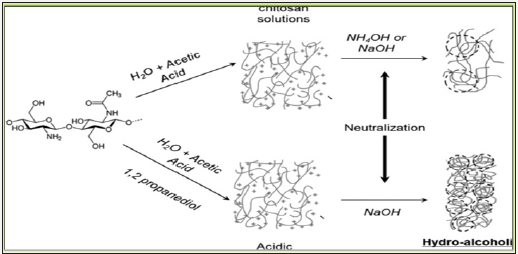
At first, chitosan obtained as cross-linked flakes that were converted into hydrogels by dissolving in 5% aqueous acetic acid and this pH inversion collision affected hydrophilic and phobic interaction to induce spontaneous entanglement of chitosan network to self-standing microsphere gel with chitosan content 2 to 3% as dispersed in 95% water (Swain, Airoldi, 2009). This followed by coagulation in an alkaline solution which subsequently formed viscous droplets/bids which were treated with graphite residue to yield desired composite powder after drying and proper meshed/ crushing as represented in Figure 9. While chitosan’s gel drying via evaporation causes dramatic shrinkage with continuity in pore size ranging from large mesopores to macropores and lose its porosity owing moderate specific surface area. This macroporosity of developed bids is attributed to space zones of contacts between chitosan fibrils though impregnation on amino groups by dopents [13-17].
Figure 9: Preparation scheme of graphite doped chiotsan composite (scale=5mm).
Conclusion
Graphite doped chitosan composites acts as an effective sorbent for nitrate/phosphates under acidic pH. The agreeable sorption achieved at appropriate pH of the solution and strictly specified process duration. The optimal pH for sorption onto was pH 4. Further adsorption of nitrate/phosphates at pH>5 is found to be ineffective as system always tends to reach the pH value close to the zero point of charge of chitosan, i.e. pHZPC=7.6. Thus solves the issue of water neutralization after the sorption treatments. The maximal quantity of nitrate/phosphates capable to be bound by chitosan composite is over 1mmol/g. The experimental data analysis indicates to tangible competition between nitrate/ phosphate for sorption sites of adsorbent surfaces. Thus suggested to apply for treatment of solutions wherein the predominating contaminant is nitrate/phosphate as the final stages of treatment i.e., after bio-treatments.
The nitrate and phosphate adsorption capacities of silica and graphite doped chitosan bio-composites were successfully assessed for good adsorption capacities towards both nutrients. Chitosan doped graphite own relatively higher adsorption capacity for nitrate and phosphate, respectively. Adsorptive removal of nitrate and phosphate generally depends on specific adsorption sites rather than BET surface area. The adsorption isotherms of phosphate on all the adsorbents are favourable, while nitrate adsorption isotherms are slightly favourable and almost linear. Hence, the adsorptive removal of nitrate seems to be more difficult than phosphate. From kinetic analysis, it is found that the adsorption uptake of nitrate and phosphate was initially rapid but becomes slower by approaching equilibrium. In describing kinetic data, the pseudo-second-order model gives marginally better predictions than the first-order model. Thus, non-expensive and benignly prepared chitosan composites are promising adsorbents for the separation of specific nutrient like nitrates/phosphates from water.
Acknowledgement
The author is thankful to Head, Department of chemistry, R.T.M. Nagpur University, Nagpur for lab facilities, Director, VNIT, Nagpur and Director, NEERI Nagpur for providing the water sample analysis and Vice Chancellor, Nagpur University, Nagpur for sanction of a research project under University Research Project Scheme, No. Dev/RTMNURP/AH/1672 (9) on dated 24-09-2016.
References
- Aktar W, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards, Interdiscip Toxicol 2(1): 1-12.
- Howarth R, Anderson D, Cloern J, Elfring C, Hopkinson C, et al. (2000) Nutrient pollution of coastal rivers, bays, and seas. Ecology Number 7(2000): 1-17.
- Ramesh R, Clark MW (2006) Phosphorus release and retention by soils of natural isolated wetlands. International Journal of Environment and Pollution 28(3/4).
- Bandpi AM, Elliott DJ, Zazouli MA (2013) Biological nitrate removal processes from drinking water supply-a review. J Environ Health Sci Eng 11: 35.
- US EPA (1986) Risk assessment guidelines of 1986-NSCEP | US EPA. EPA/600/8-87/045. Environmental Protection Agency, Washington, USA.
- Kumar M, Puri A (2012) A review of permissible limits of drinking water. Indian J Occup Environ Med 16(1): 40-44.
- Loon AJ, Botterweck AA, Goldbohm RA, Brants HA, Klaveren JD, et al. (1998) Intake of nitrate and nitrite and the risk of gastric cancer: a prospective cohort study. Br J Cancer 78(1): 129-135.
- de-Bashana LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997- 2003). Water Res 38(19): 4222-4246.
- Bhatnagar A, Sillanpää M (2011) A review of emerging adsorbents for nitrate removal from water, chemical engineering journal 168(2): 493- 504.
- Jung KW, Hwang MJ, Ahn KH, Ok YS (2015) Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int J Environ Sci Technol 12(10): 3363- 3372.
- Öztürk N, Bekta ET (2004) Nitrate removal from aqueous solution by adsorption onto various materials. Journal of Hazardous Materials 112(1-2): 155-162.
- Rabih S, Khaled B, Safia H (2007) Adsorption of phosphate and nitrate anions on ammonium-functionalized MCM-48: effects of experimental conditions. Journal of Colloid and Interface Science 311(2): 375-381.
- Shrimali M, Singh KP (2001) New methods of nitrate removal from water. Environmental Pollution 112(3): 351-359.
- Kim JY, Balathanigaimani MS, Moon H (2015) Adsorptive removal of nitrate and phosphate using MCM-48, SBA-15, chitosan, and volcanic pumice, water, air, & soil pollution 226(12): 1-11.
- Chatterjee S, Woo SH (2009) The removal of nitrate from aqueous solutions by chitosan hydrogel beads. Journal of Hazardous Materials 164(2-3): 1012-1018.
- Henze M (1995) Nutrient removal from wastewater. In: Harris R (Ed.), New World Water. Sterling Publications Limited, London, UK, pp. 114- 115.
- Jóźwiak T, Filipkowska U, Szymczyk P, Mielcarek A (2014) Application of cross-linked chitosan for nitrate nitrogen removal (V) from aquesous solutions. Progress on Chemistry and Application of Chitin and Its Derivatives 19: 41-52.
© 2018 Rajendra S Dongre. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






