- Submissions

Full Text
Research & Development in Material Science
Fullerene Functionalized Thiophene Derivative as an Acceptor Material for Organic Photovoltaics
Ranjith Kottokkaran*, Arun D Rao, Vinila NV and Praveen C Ramamurthy
Department of Materials Engineering, Indian Institute of Science, India
*Corresponding author: Ranjith Kottokkaran, Department of Materials Engineering, Indian Institute of Science, Bangalore, India
Submission: August 17, 2017; Published: October 20, 2017

ISSN : 2576-8840Volume1 Issue4
Abstract
A functionalized fulleropyrrolidine derivative of 4, 7-di (thiophen-2-yl)-2, 1, 3-benzothiadiazole (F-DTBT) was designed and synthesized by 1, 3-dipolar cycloaddition reaction. This thermally stable fullerene derivative exhibits an absorption peak at 425nm along with the characteristic peak of fullerene. This functionalized derivative shows deeper HOMO level, -6.16eV and higher LUMO level -3.6eV compared to C60PCBM. Bulk heterojunction solar cells were fabricated using F-DTBT as the acceptor and P3HT as the donor material. The preliminary result shows a power conversion of efficiency of 1.1% with a Voc of 0.61V, a Jsc of 6.1mAcm-2 and a fill factor of 29%. This functionalized DTBT with fullerene could be a stronger acceptor moiety compared to PCBM and could be a promising candidate for organic photovoltaics
Keywords: Fullerene functionalization; Bulk heterojunction; Organic photovoltaics
Introduction
Organic photovoltaic devices (OPV) have become a most active research area due to their promising advantages such as ease of processing, low cost synthesis and clean-renewable energy sources [1]. Introduction of Bulk hetero junction solar cells (BHJ) in OPV leads to an improved power conversion efficiency (PCE), due to its interpenetrating network structure of active layer [2,3]. The active layer of BHJ solar cell is comprised of a donor and acceptor material. From the material point of view, the development of OPVs is mainly due to the development in the synthesis of new donor materials for the active layer. The progress in acceptor moiety is relatively low. Fullerene derivatives have gained much interest as an acceptor material due to its structural uniqueness and electronegative character [4]..
Fullerenes and their derivatives exhibit non linear optical properties [5,6]. The photo physical properties of the fullerenes can be tailored by the chemical modification of the buckyball [7-10]. Fullerene as such cannot be used as an acceptor material for OPVD due to its less solubility in common organic solvents. [6,6]-phenyl- C60-butyric acid methyl ester (PC60BM)is one of the chemically modified fullerenes gained much interest as the acceptor material in OPV due to its low lying lowest unoccupied molecular orbital (LUMO), high electron mobility and compatibility with most of the donor materials, which are soluble in most of the common organic solvents [11]. Even though these fullerene derivatives are well explored in the field of OPV, researchers have been trying to replace PC60CBM with its higher fullerene analogue C70PCBM [12,13] in order to improve the efficiency of the devices. More over these fullerene derivatives are also used in organic field effect transistors [14].
The main drawback of C60PCBM is its insufficient absorption in the visible region. This arises due to the structural symmetry of the C60PCBM which forbids low-energy transitions. One of the strategy to overcome these drawbacks is functionalization of fullerene to extend the absorption spectra to UV-V is region [15,16]. Several approaches have been introduced to modify C60PCBM chemically such as substitution of phenyl group to other aromatic groups or varying alkyl chain length etc [10,17]. Other than these C60PCBM like molecules, indene-fullerenes, endohedrall fullerenes etc. have been synthesized for various organic electronics applications [16,18]. Kim et al. [19] reported carbazole substituted fullerenes such as fulleropyrrolidine derivative.
Fulleropyrrolidine derivatives can be used for OPV application due to their high electron affinity and good charge transportability [20,21]. Here in this work fulleropyrrolidine derivative of 4, 7-di (thiophen-2-yl)-2, 1, 3-benzothiadiazole (DTBT) was synthesized by 1, 3-dipolar cycloaddition reaction. DTBT units were widely used as electron accepting units in organic electronics. Functionalization of DTBT with fullerene could result a strong acceptor unit that can be used for OPV application. Structural, optical, and electrical characterization of the functionalized DTBT was carried out and photovoltaic property was evaluated by fabricating a PV device with P3HT as the donor.
Experimental section
Materials
O-phenylenediamine, Thiophene, tributylchlorostannane, Tetrakis (triphenylphosphine) palladium (Pd(PPh3)4), N-methylglycien, triphenylphosphine and tetrabutylammoniumhexafluorophosphate (TBAPF6) were purchased from Sigma Aldrich and used without further purification. n-Bun-BuLi solution (1.6M in hexane) was purchased from across chemicals and used as such. Solvents and other reagents were purchase locally and distilled before use.
Synthesis
Synthesis of 2,1,3-benzothiadiazole: o-phenylenediamine (1g, 9.2472mmol) and triethylamine (3.7g, 36.9890mmol) in 30mL DCM were taken in a round bottom flask. 2.2 gram (18.4950mmol) of thionyl chloride was added drop wise to the reaction mixture at 0 °C. Temperature of the reaction mixture was maintained at 0 °C for 30min, followed by refluxing for 5h at 50 °C. The solvent was removed using a rotary evaporator and quenched the reaction mixture with DI water. A pH of 1 was achieved using concentrated hydrochloric acid (HCl). Product was extracted with dichloromethane (3×50mL) and dried over sodiumsulphate (NaSO4). Further the product was purified by column chromatography using hexane/ethyl acetate as eluting solvent. Yield= 87% (Figure 1).
Figure 1: 1HNMR spectrum of 2,1,3-benzothiadiazole.
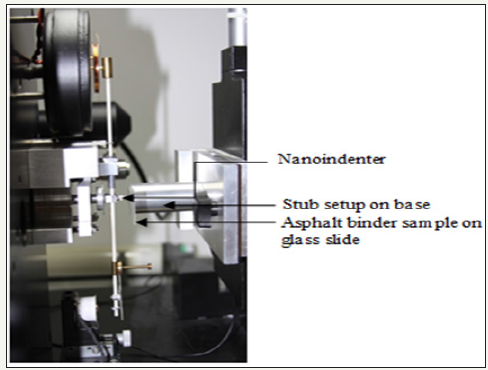
Figure 2: 1HNMR spectrum of 4,7-dibromo-2,1,3-benzothiadiazole.
Synthesis of 4, 7-dibromo-2, 1, 3-benzothiadiazole: A solution of Br2 in HBr (5mL) was added slowly to a mixture of 2, 1, 3-benzothiadiazole (0.5g, 3.6717mmol) and 10mL of HBr (48%) at 0 °C. After stirring the reaction mixture at room temperature for 3h, refluxed for 6h at 110 °C, reaction mixture was cooled to room temperature and excess Br2 was removed by saturated solution of sodium bisulphite. The mixture was filtered and the precipitate was washed with copious amount of DI water and cold diethyl ether. The purification of the product was carried out by recrystallization from ethanol. Yield=81% (Figure 2).
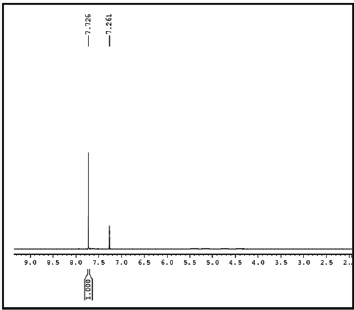
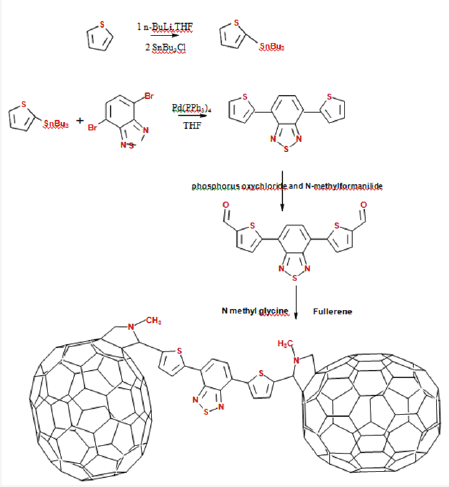
Synthsis of tributyl (thiophen-2-yl) stannane: Thiophene (2.0g, 23.7699mmol) and 15mL freshly distilled dry THF were taken under inert atmosphere. 16.2mL (26.1469mmol) of n-BuLi 1.6M in hexane was added drop wise at 0 °C and for 1h at 0 °C. The reaction mixture was then cooled down to -78 °C and tributylchlorostannane (6.4mL, 23.7699mmol) was added. The mixture was stirred for another 12h at room temperature. The reaction mixture was quenched with 100mL of ice cooled water, and the organic layer was extracted with diethyl ether (3×30mL). The solvent was removed from the combined organic layer by rotary evaporation and obtained the product as brown oil (88%). The crude product was used in next step without further purification (Figure 3).
Figure 3: 1HNMR spectrum of tributyl(thiophen-2-yl)stannane.
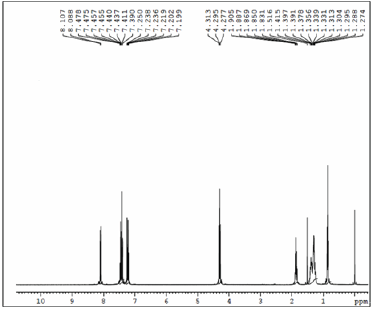
Figure 4: 1HNMR spectrum of DTBT.
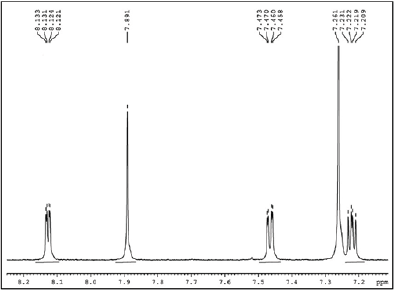
Synthesis of DTBT: 5.3593mmol) were taken in dry toluene (15mL). After purging the reaction mixture for 10 minutes with nitrogen, catalyst Pd (PPh3)4 (4mol%) was added. The reaction mixture was heated to 80 °C and stirred for 24h. After cooling to room temperature, the reaction mixture was poured in to cold water (100mL). The organic layer was extracted with dichloromethane (3×50mL) and the product was purified by column chromatography. Yield (72%) (Figure 4).
Synthesis of 5-[7-(thiophen-2-yl)-2,1,3-benzothiadiazol- 4-yl]thiophene-2-carbaldehyde: 5-[7-(thiophen-2-yl)-2,1,3- benzothiadiazol-4-yl]thiophene-2-carbaldehyde as synthesized by the Vilsmeier reaction. Vilsmeier reagent was prepared by adding POCl3 (0.4mL, 4.32mmol) drop wise to DMF (0.32mL, 4.32mmol) at 0 °C under nitrogen atmosphere. The system was allowed to stir for 15 min at the same temperature. DTBT (0.5g, 1.6643 mmol) in 5mL of dichloroethane was then added to the reaction mixture and refluxed for 12h. The reaction mixture was quenched by ice cold water and stirred for 30 min. Organic layer was extracted using DCM and dried over anhydrous Na2SO4. After removing the solvent under reduced pressure, the product was purified by column chromatography. (Yield= 67%) (Figure 5).
Figure 5: 1HNMR spectrum of 5-[7-(thiophen-2-yl)-2,1,3- benzothiadiazol-4-yl]thiophene-2- carbaldehyde.
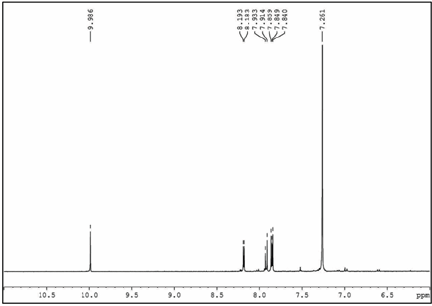
Figure 6: 1HNMR spectrum of F-DTBT.
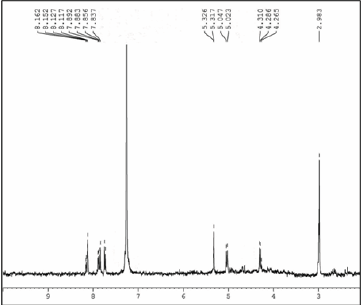
Functionalization of fullerene on DTBT (F-DTBT): 5-[7-(thiophen-2-yl)-2,1,3-benzothiadiazol-4-yl]thiophene-2- carbaldehyde (0.3g, 0.0913mmol) was added to a dried two neck round bottom flask containing 40mL of chlorobenzene. The solution was purged with nitrogen gas for 10 min. C60 (1.96g. 0.2739mmol) and N-methylglycien (0.82g, 0.9134mmol) was then added to the solution. The system was further purged with nitrogen gas for another 15 min. The reaction mixture was heated to reflux for 24h. After cooling the system to room temperature, chlorobenzene was removed using rotary evaporator under reduced pressure. The crude product was purified by column chromatography on silica gel using toluene and carbon disulfide as the eluting solvents (Figure 6).
Results and Discussion
Synthesis was started with the synthesis of 2,1,3-benzothiadiazole (BT) from o-phenylenediamine. 4,7-dibromo-2,1,3-benzothiadiazole was synthesized by the bromination of 2,1,3-benzothiadiazole with Br2 in HBr. Tributyl(thiophen-2-yl)stannane was obtained by the stannyaltion of thiophene with SnBu3Cl and n-BuLi as the reagents. Synthesis of DTBT was carried out by the Pd (0) catalyzed Stille coupling reaction of 4,7-dibromo-2,1,3-benzothiadiazole and tributyl(thiophen- 2-yl)stannane. Fullerene functionalized DTBT (F-DTBT), a kind of fulleropyrrolidinederivative, was synthesized through the 1,3-dipolarcycloaddition reaction of 5- [7-(thiophen-2-yl)-2,1,3- benzothiadiazol-4-yl]thiophene-2-carbaldehyde with C60.
Figure 7: UV-visible absorption spectrum of F-DTBT.
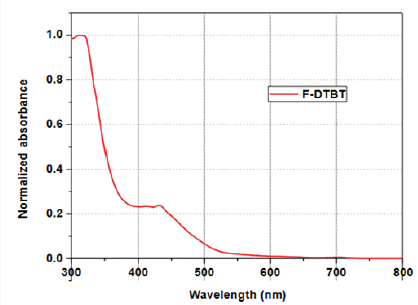
Optical studies of the F-DTBT were carried out using UVvisible spectroscopy. Absorption spectrum of F-DTBT in dilute chlorobenzene solution is as shown in (Figure 7). An absorption peak is observed at about 320nm, which is the common absorption peak for C60 molecules. The absorption peak at about 425nm has been observed after the incorporation of DTBT on fullerene. This absorption hump suggests that functionalization of PC60BM with DTBT resulted in the broadening of absorption spectra. Optical band gap of the molecule is obtained from the absorption onset and is determined as 2.38eV.
Electronic structure of F-DTBT was determined using cyclic voltammetry (CV). CV experiment was carried out in acetonitrile solvent using TBAPF6 as the supporting electrolyte. Cyclic voltammogram of F-DTBT is as shown in (Figure 8). F-DTBT shows three quasi reversible one electron reduction waves during negative sweep. The reduction peaks are observed at about -1.10V, -1.44V and -1.77V. By considering the onset of first reduction, LUMO energy level was calculated. The onset of first reduction potential of F-DTBT is observed at -0.80V with a LUMO level of -3.6eV. HOMO level of F-DTBT was calculated by adding the optical band gap with LUMO level and is determined as -6.16eV. It is reported that, the HOMO-LUMO levels of C60PCBM is -6.1eV and-3.78eV respectively. Hence, F-DTBT has a deeper HOMO level and higher LUMO level than that of C60PCBM. This higher LUMO of DTBT functionalized fullerene will results in well energy level matching with the donor molecules of high HOMO. Thermal properties of F-DTBT were studied by thermo gravimetric analysis. TGA thermogram of F-DTBT is as shown in (Figure 9). 10% weight loss of F-DTBT occurs at about 315 °C. BHJ solar cells were fabricated using F-DTBT as the acceptor material and P3HT as the donor material for active layer. The configuration of the fabricated solar cell is ITO/PEDOT: PSS/P3HT: F-DTBT (1:1 wt%)/Al (Figure 10). The transparent ITO coated glass plates were cleaned by ultra sonication with acetone, DI water and isopropanol respectively. About 30nm of PEDOT: PSS was spin coated on the cleaned ITO glass plates at 5000rpm for 60s. These spin coated samples were then annealed at 150 °C for 30 min on a hot plate. Active material for the device was obtained by dissolving P3HT and F-DTBT in chlorobenzene (15mgmL-1, 1:1 weight ratio) with overnight stirring. The active material was spin coated at 1500rpm for 10s and dried at 130 °C for 15min. Aluminum electrodes were deposited on the active layer using a multi source thermal evaporator at a base pressure of 5×10-6Torr.
Figure 8: Cyclic voltammogram of F-DTBT.
Figure 9: TGA thermogram of F-DTBT.
Figure 10: Device architecture of P3HT: F-DTBT solar cell.
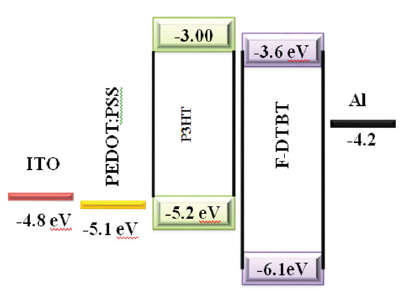
Figure 11: FM images of P3HT: F-DTBT film.
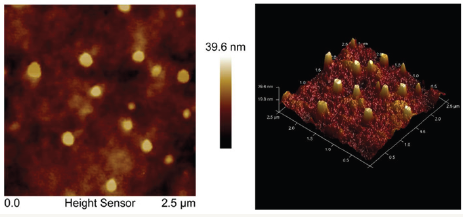
Figure 12: PV characteristics of P3HT: F-DTBT BHJ solar cell.
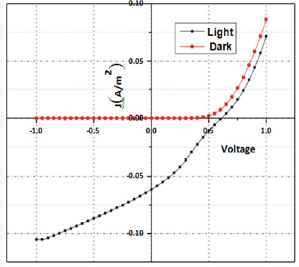
AFM image of the top surface of a BHJ film on ITO substrate is as shown in (Figure 11). It shows a mean roughness of 4.3nm. The bright features in this phase image, which correspond to bumps in topography, indicate that FDTBT-rich domains are present at the top surface. It also shows that it is not a homogeneous dispersion having a mean diameter of 92±33 nm which could impact the performance of the device. For an efficient device, acceptor material should form a homogeneous dispersion on the surface such that can be in contact with the cathode for efficient extraction of electrons from the device. This may be one of the reasons for the moderate performance of the device. PV characteristics of the fabricated device are as shown in (Figure 12 & 13). Short circuit current density and open circuit voltage of the device are 6.1mAcm- 2 and 0.61V respectively. Fill factor of the fabricated PV device is determined as 29%. Power conversion efficiency of the fabricated photovoltaic device is obtained as 1.1%. Further optimization of device architecture using hole transport layers and exciton blocking layers will enhance the performance of the solar cells.
Figure 13: Synthesis scheme of F-DTBT.
Conclusion
A functionalized fullerene derivative, F-DTBT was designed and synthetic routes were developed. This thermally stable fullerene derivative shows absorption peaks around 320 and 425nm. In addition to the C60 absorption peak, another absorption peak at 425 is observed after the functionalization. Electrochemical studies show that, these functionalized materials have higher LUMO levels compared to the C60. BHJ solar cells were fabricated using F-DTBT as the acceptor and P3HT as the donor materials and PV parameters are evaluated. With F-DTBT as an acceptor material, PCE of 1.10% with a Voc of 0.61V, a Jsc of 6.1mAcm-2 and a fill factor of 29% were obtained.
Acknowledgment
This work is funded by DST No SB/SR/S3/ME/51/2012 and technical support from Advanced Facility for Microscopy and Microanalysis (AFMM), Indian Institute of Science (IISc).
References
- Lu L, Zheng T, Wu Q, Schneider AM, Zhao D, et al. (2015) Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem Rev 115(23): 12666- 12731.
- Brabec CJ, Zerza G, Cerullo G, Silvestri SD, Luzzati S, et al. (2001) Tracing photoinduced electron transfer process in conjugated polymer/fullerene bulk heterojunctions in real time. Chem Phy Lett 340(3-4): 232-236.
- Kraabel B, Hummelen JC, Vacar D, Moses D, Sariciftci NS, et al. (1996) Subpicosecond photoinduced electron transfer from conjugated polymers to functionalized fullerenes. Chem Phys 104: 4267.
- Cui C, Li Y, Li Y (2017) Fullerene derivatives for the applications as acceptor and cathode buffer layer materials for organic and perovskite solar cells. Adv Energy Mater 7(10).
- Diederich F, Thilgen C (1996) Covalent Fullerene Chemistry Science 271(5247): 317-324.
- M. Prato (1997) [60] Fullerene chemistry for materials science applications. J Mater Chem 7(7): 1097-1109.
- Li C, Chen Y, Wang Y, Iqbal Z, Chhowalla M, et al. (2007) A fullerenesingle wall carbon nanotube complex for polymer bulk heterojunction photovoltaic cells. J Mater Chem 17(23): 2406-2411.
- Yu D, Park K, Durstock M, Dai L (2011) Fullerene-Grafted Graphene for Efficient Bulk Heterojunction Polymer Photovoltaic Devices. J Phys Chem 2(10): 1113-1118.
- Mi DB, Kim JH, Xu F, Lee SH, Cheol YS, et al. (2011) Synthesis and characterization of a novel fullerene derivative for use in organic solar cells. Sol Energy Mater Sol Cells 95: 1182-1187.
- Zhu D, Li Y, Wang S, Shi Z, Du C, et al. (2003)Design, synthesis and properties of functional materials based on fullerene. Synth Met 133(134): 679-683.
- Meng X, Jiang L, Shu C, Wang C (2012) Chin Sci Bull Chin Version 57: 3437.
- He Y, Li Y (2011) Fullerene derivative acceptors for high performance polymer solar cells. Phys Chem Chem Phys 13(6): 1970-1983.
- Li CZ, Yip HL, Jen AKY (2012) Functional fullerenes for organic photovoltaics. J Mater Chem 22(10): 4161-4177.
- Nam SY, Park EY, Kim TD, Cho S, Park JG, et al. (2011) Solutionprocessable fullerene derivatives for organic photovoltaics and n-type thin-film transistors. Curr Appl Phys 11(2): e44-e48.
- Zhang Y, Yip HL, Acton O, Hau SK, Huang F, et al. (2009) A Simple and Effective Way of Achieving Highly Efficient and Thermally Stable Bulk-Heterojunction Polymer Solar Cells Using Amorphous Fullerene Derivatives as Electron Acceptor. Chem Mater 21(13): 2598-2600.
- Backer SA, Sivula K, Kavulak DF, Fréchet JMJ (2007) high efficiency organic photovoltaics incorporating a new family of soluble fullerene derivatives. Chem Mater 19(12): 2927-2929.
- Sharma GD, Mikroyannidis JA, Sharma SS, Justin Thomas KR (2012) Bulk heterojunction organic photovoltaic devices based on small molecules featuring pyrrole and carbazole and 2-(4-nitrophenyl) acrylonitrile acceptor segments as donor and fullerene derivatives as acceptor. Dyes and Pigments 94(2): 320-329.
- Wang N, Bao X, Yang C, Wang J, Woo HY, et al. (2013) Design and synthesis of indole-substituted fullerene derivatives with different side groups for organic photovoltaic devices. R Yang Org Electron 14(2): 682-692.
- Kim HU, Mi D, Kim JH, Park JB, Yoon SC, et al. (2012) Carbazole-containing fullerene derivatives for P3HT-based bulk-heterojunction solar cells. Sol Energy Mater Sol Cells 105: 6-14.
- Li Y, Zheng D, Xu J, Mao Z, Yang J, et al. (1997) Synthesis of fulleropyrrolidine derivatives of C60. Chin Sci Bull 42(14): 1180-1184.
- Mi D, Kim HU, Kim JH, Xu F, Jin SH, et al. (2012) Synthesis of a soluble fulleropyrrolidine derivative for use as an electron acceptor in bulkheterojunction polymer solar cells. Synth Met 162(5-6): 483-489.
© 2017 Ranjith Kottokkaran, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






