- Submissions

Full Text
Research & Development in Material Science
Corrosion Protection Polybutadiene-Coated Mild Steel in Marine Water by Nanocoating and Filler Compounds
Rajesh Kumar Singh*, Shabana Latif and Manjay Kumar Thakur
Department of Chemistry, JP University, India
*Corresponding author: Rajesh Kumar Singh, Department of Chemistry, Jagdam College, JP University, Chpara, India
Submission: August 01, 2017; Published: September 22, 2017

ISSN : 2576-8840Volume1 Issue1
Abstract
Mild steel is economical metal so it is used in different appliances of industries, railways, bridges, construction works and marine water. Marine water produces corrosive environment for this metal. Marine water is saline in character so it corrodes mild steel. Polybutadiene is coated on the surface of mild steel for corrosion protection. But this coating does not provide sufficient protection of base metal. Marine water is major absorber of CO2. It converts CO2 into H2CO3. Saline water and H2CO3 interacts with polybutadiene-coated mild steel and they exhibit chemical and electrochemical reactions. This chemical reaction produces swelling and dissolving corrosion and produce disbonding between carbon and carbon of polybutadiene. These corrosive agents show osmosis or diffusion process and inter inside the base metal and develop corrosion cell. Metal generates corrosion reaction which produces several forms of corrosion like galvanic, pitting, crevice, stress, intergranular, blistering and embrittlement. For the protection from such types of corrosions synthesized organic compounds octahydrodibenzo[a,d][8]annulene-5,12-dioxime was used and this compounds was nanocoated on the surface of polybutadiene-coated mild steel. During nanocoating used compound developed lot of porosities on the surface of polybutadiene-coated mild steel and they were blocked by the use of filler materials ZnS. The corrosion rate of material was calculated by gravimetric and potentiostat. Composite barrier formation was analyzed by activation energy, heat of adsorption, free energy, enthalpy and entropy. The results of surface coverage area and coating efficiency were indicated that compound octahydrodibenzo[a,d][8]annulene-5,12-dioxime and ZnS have good coating and filling properties.
Introduction
Corrosion is just like diabetes for materials. Corrosion control of materials is major problems. Develop nation’s expense 5% their GNP for corrosion protection and repairing of materials. Corrosion occurs in metals, metalloids, ceramics, polymers, biometals, building materials and other type’s metallic and nonmetallic equipments. There are several methods are applied for corrosion mitigation of materials [1] as per materials nature and environments. Generally must of metallic industries use metallic coatings but such types of coating do not provide good results in corrosive medium [2]. Now days some industries apply polymeric coating [3] for safe base metal in hostile atmosphere [4]. This coating does not provide protection [5] of base and its own. Disintegrations occur in base metal [6] and polymeric material [7]. Paint coating [8] is doing for building materials [9] and metal corrosion protection [10] in unfriendly atmosphere [11] but it is not protect itself and coating materials [12] for the attack of pollutants [13]. Organic [14] and inorganic inhibitors [15] are a very useful in acidic, basic and moist oxygen environment [16]. These inhibitors do not give good results after certain period. There are several types of inhibitors [17] like anodic, cathodic and mixed [18] of types use as nature environments of pollutants. They do not provide satisfactory results in against pollutants [19]. Nanocoating techniques [20] are a very suitable method for corrosion protection materials in any type’s corrosive ambient. Nanocoating substances forms composite thin film barrier, top layer coating, thermal barrier coating, conversion coating and nano scale structural change coatings on surface of base materials as per materials applications in different fields [21] to safe it’s against heat, light, temperature, particulates, acidic, basic, salt, marine atmosphere, flues gases and biological environment [22]. Such types of coating produce lot of porosities and corrosive agents are entered inside by osmosis or diffusion process and produce interior and exterior corrosion. In this research work octahydrodibenzo[a,d][8]annulene-5,12-dioxime is used a nanocoating material and porosities are blocked by filler ZnS. These materials create composite barrier and it works repeller against corrosive pollutants.
Experimental
Polybutadiene-coated mild steel kept in marine water and water sample was taken from Marina beach of Chennai (Tamil Nadu). Polybutadine coated-mild steel (5X10Xwas in dipped into sea water and the corrosion rate was determined gravimetric methods at 283, 293,303,313 and 333 0K temperatures and time mentioned in given temperatures 2,5,8,11 and 14 days. The samples were nanocoated with octahydrodibenzo[a,d][8]annulene-5,12- dioxime and immersed into sea water and corrosion rate recorded at above mentioned temperatures and days. Nanocoated samples were coated by ZnS filler and dipped into sea water to calculate the corrosion rate given temperatures and days. The corrosion potential, corrosion current and corrosion current densities were calculated by potentiostat technique. For these results pt electrode used as reference electrode, calomel as auxiliary electrode and polybutaine-coated mild steel sample electrode. Nanocoated compound octahydrodibenzo[a,d][8]annulene-5,12-dioxime was synthesized by given methods as:
Synthesis of 4-chloro-1, 2-dihydonaphthalene
into cold solution of benzene (50gm) containing PCl5 (30gm), the reaction mixture was stirred for one hour. The reaction mixture was quenched with NaHCO3 and did workup with diethyl ether. The solvent evaporated with rotator vapour. The product was purified by silica gel column chromatography and produced 89% 4-chloro-1, 2-dihydonaphthalene (Figure 1-4).
Figure 1: Synthesis of 4-chloro-1, 2-dihydonaphthalene.

Figure 2: Physical properties of 4-chloro-1, 2-dihydronaphthalene.
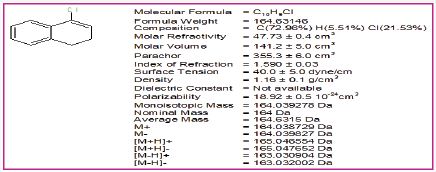
Figure 3: H1NMR of 4-chloro-1, 2-dihydronaphthalene.
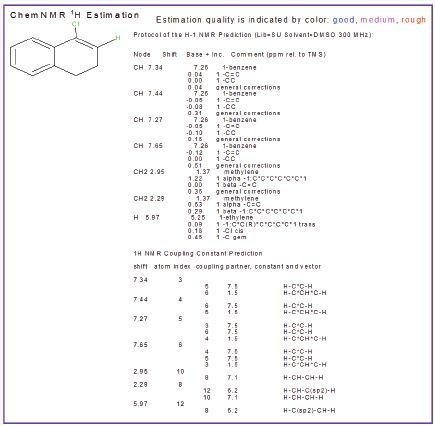
Figure 4: XRD of 4-chloro-1,2-dihydronaphthalene.
Synthesis of 1,2-didehydro-3,4-dihydronaphthalene
4-Chloro-1,2-dihydronaphthalene (10gm) kept in two neck round bottle flask and potassium t-butaoxide (25gm) dissolved in THF solution. This solution poured into 4-Chloro-1,2- dihydronaphthalene and reaction temperature 00C. The reaction was mixture stirring four hours after completion reaction added cyclohexene as trapping agent and again stirring reaction more two hours. After work up got adduct 90% of 1,2-didehydro-3,4- dihydronaphthalene (Figure 5 & 6).
Figure 5: Synthesis of 1,2-didehydro-3,4-dihydronaphthalene.
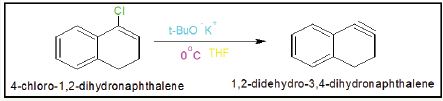
Figure 6: Physical properties of 1,2-didehydro-3,4-dihydronaphthalene.
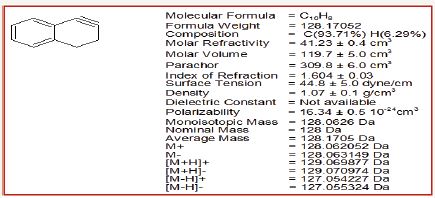
Synthesis of benzo-decahydrobiphenylene
When 1,2-didehydro-3,4-dihydronaphthalene was used with cyclohexene, it was trapped by 1,2-didehydro-3,4- dihydronaphthalene to yield benzo-decahydrobiphenylene (Figure 7-9).
Figure 7: Synthesis of benzo-decahydrobiphenylene.
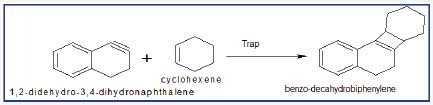
Figure 8: Physical properties of benzo-decahydrobiphenylene.
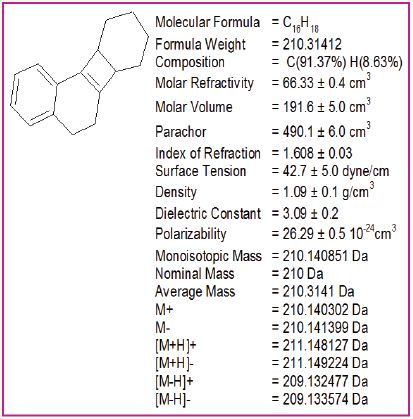
Figure 9: H1NMR of benzo-decahydrobiphenylene.

Synthesis of octahydrodibenzo[a,d][8]annulene-5,12- dione
TAdduct (20gm) oxidized into benzo-decahydrobiphenylene with addition of NaIO4 (10gm) and RuO2 (15g) in the presence of solvent CH3CN and CCl4. The reaction was quenched with H2O and after workup 87% yield of octahydrodibenzo[a,d][8]annulene- 5,12-dione was obtained Figure 10-12.
Figure 10: Synthesis of octahydrodibenzo[a,d][8]annulene- 5,12-dione.
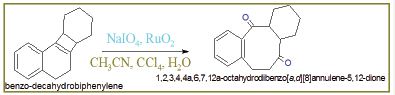
Figure 11: Physical properties of octahydrodibenzo [a,d][8]annulene- 5,12-dione
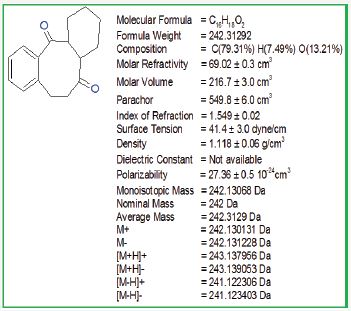
Figure 12: H1NMR of octahydrodibenzo [a,d][8]annulene-5,12- dione.
Synthesis of octahydrodibenzo[a,d][8]annulene-5,12- dioxime
Octahydrodibenzo[a,d][8]annulene-5,12-dione (30g), hydroxylamine hydrochloride (50g) and 135ml of dry ethanol were taken and adding 70ml of pyridine and refluxing reaction mixture for two hours. Solvent was removed by use of rotator vapour and water was added in reaction mixture and after cooling the reaction by ice, solution was stirred until oxime crystallized. Solid was filtered and washed with a little water and then dried. The product was recrystallized with ethanol and 71% yield of octahydrodibenzo[a,d][8]annulene-5,12-dioxime was obtained Figure 13 & 14.
Figure 13: Synthesis of octahydrodibenzo[a,d][8]annulene- 5,12-dioxime.
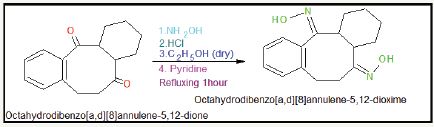
Figure 14: Physical properties of octahydrodibenzo [a,d][8]annulene- 5,12-dioxime.
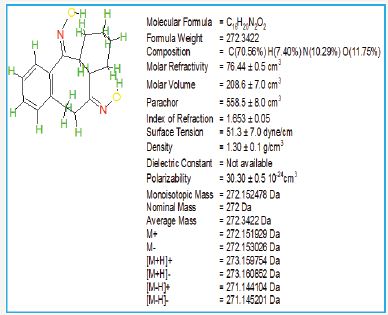
Results and Discussion
Marine water generates hostile environment for polybutadienecoated mild steel. Corrosion is control in such hostile environment by the use of nanocoating and filler materials. Polybutadienecoated mild steel corrosion rates were studied in marine water environment at 283, 293, 303, 313 and 323 0K temperatures after interval of 2, 5, 8, 11 and 14days with the help of gravimetric method equation K(mmpy) = 13.56 X(W/DAt) (where W=weight loss of test coupon expressed in kg, A=area of test coupon in square meter, D=Density of the material in kg. m-3) and their values recorded in Table 1. Similarly, the samples of polybutadiene-coated mild steel nanocoated with octahydrodibenzo[a,d][8]annulene- 5,12-dioxime and ZnS filler were immersed into marine water and corrosion rate was calculated on above mentioned temperatures and days and their values were mentioned in Table 1, Figure 15 plotted between corrosion rate K(mmpy) versus times (t) in days which produced straight line and it indicated that corrosion rate of polybutadiene-coated mild steel increased without coating and their values were reduced after nanocoating and filler compounds. The results of Table 1 were shown that the corrosion rate of material is reduced by the action of nanocoating and filler compounds. The octahydrodibenzo[a,d][8]annulene-5,12-dioxime is an electronic rich compound and large molecular weight so it is suitable for nanocoating materials. Nanocoating compound is coordinated its electron to ZnS filler thus they can form strong barrier on the surface of polybutadiene-coated mild steel. This barrier stops osmosis or diffusion process of saline water.
Table 1: Corrosion rate of polybutadine-coated mild steel nanocoated with octahydrodibenzo [a,d][8] annulene-5,12-dioxime[NC(1)] and ZnS filler in marine water.
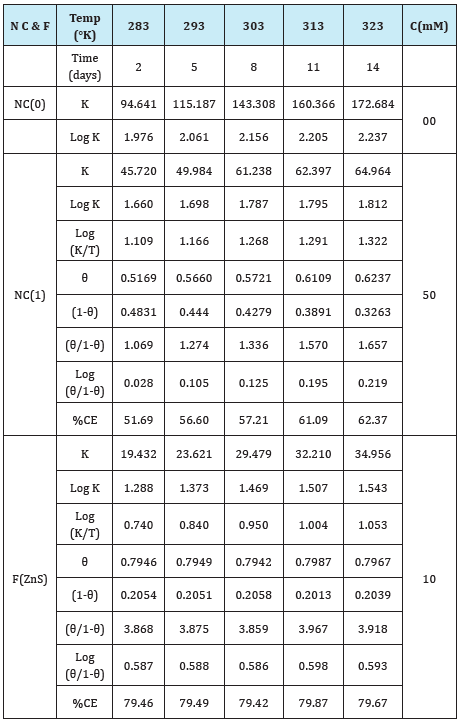
Figure 15: K (mmpy) Vs t (days) for nanocoating of NC(1) & ZnS filler on polybutadiene-coated mild steel.
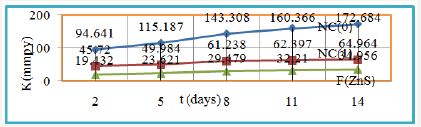
Figure 16: log K Vs 1/T for nanocoating NC (1) & ZnS filler on polybutadiene-coated mild steel.

Studied the effect of temperature on the polybutadiene-coated mild steel at mentioned above temperatures and their results were written in Table 1, it observed that corrosion rate of material increased without nanocoating but its values were reduced with nanocoating and filler compounds such types trends clearly noticed in Figure 16 which plotted between log K versus 1/T found to be a straight line. Nanocoating and filler compounds were formed a stable barrier with on the surface of polybutadiene-coated mild steel. This barrier has thermal stability and suppressed the attack of Cl- ions. Nanocoating and filler materials were developed composite barrier i.e. stable in saline water as temperature increased.
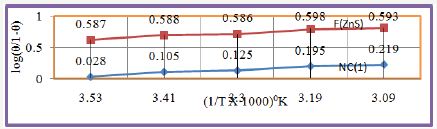
Figure 17: log (θ/1-θ) Vs 1/T for nanocoating of NC (1) & ZnS filler on polybutadiene-coated mild steel
The surface coverage area (θ) of nanocoating compound octahydrodibenzo[a,d][8]annluene-5,12-dioxime and ZnS filler was calculated by equation θ=(1-K/Ko) (where K is the corrosion rate before coating and Ko is the corrosion rate after coating) and their values were mentioned in Table 1. Figure 18 plotted between surface coverage (θ) versus temperature (T) which indicated that filler compound covered more surface area with respect of nanocoating compound octahydrodibenzo[a,d][8]annluene- 5,12-dioxime. The low dose of nanocoating and filler compounds were occupied more surface coverage area as temperatures
Figure 18: θ Vs T for nanocoating of NC(1) & ZnS filler on the polybutadiene-coated mild steel.

The surface coverage area (θ) of nanocoating compound octahydrodibenzo[a,d][8]annluene-5,12-dioxime and ZnS filler was calculated by equation θ=(1-K/Ko) (where K is the corrosion rate before coating and Ko is the corrosion rate after coating) and their values were mentioned in Table 1. Figure 18 plotted between surface coverage (θ) versus temperature (T) which indicated that filler compound covered more surface area with respect of nanocoating compound octahydrodibenzo[a,d][8]annluene- 5,12-dioxime. The low dose of nanocoating and filler compounds were occupied more surface coverage area as temperatures were increased. These results were given information that as temperatures were risen nanocoating and filler compounds were accommodated more surface areas.
Figure 19: %CE Vs T for nanocoating of NC(1) & ZnS filler on polybutadiene-coated mild steel.
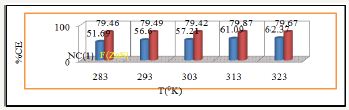
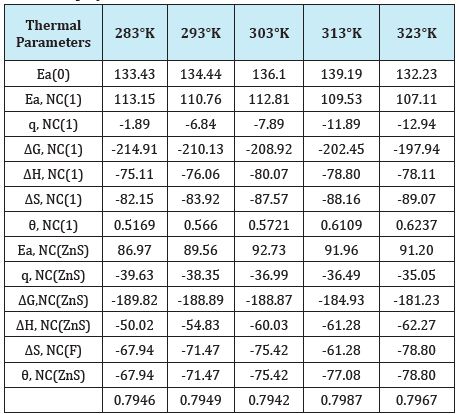
The percentage coating efficiency for octahydrobenzo [a,d] [8]annulene-5,12-dioxime and ZnS filler were calculated by equation, %CE = (1-K/Ko) X 100 (where CE=coating efficiency , K=Corrosion rate after coating, Ko=corrosion rate before coating) and their values were given in Table 1. Figure 19 plotted between %CE (percentage coating efficiency) versus T (temperature) indicated that filler compound enhanced percentage coating efficiency with respect of nanocoating compound. Nanocating and filler compounds were electron rich compound so they have more binding capacities. Activation energy of polybutadiene-coated mild steel, nanocoating compound octahydrodibenzo[a,d][8]annulene- 5,12-dioxime and ZnS filler were determined by Arrhenius equation, d/dt (log K)=Ea/RT2 (where T is temperature in Kelvin, R is universal gas constant and Ea is the activation energy of the reaction) and Figure 16 which plotted between log K versus 1/T. The calculated values of activation energies were mentioned in Table 2. Polybutadiene-coated mild steel produced high activation energies at different temperatures in marine water whereas nanocoating and filler compounds exhibited lower activation energy. The results of activation energies were shown that nanocoating and filler compounds adhered on the surface of polybutadiene-coated mild steel by chemical bonding Table 2. Thermal parameters of nanocoating compound octahydrodibenzo [a,d][8]annulene-5,12- dioxime and ZnS filler coated on polybutadiene-coated mild steel in marine water heat of adsorption of nanocoating compound octahydrodibenzo[a,d][8]annulene-5,12-dioxime and ZnS filler were calculated by Langmuir equation, log (θ/1-θ) = log (A .C) - (qads/ 2.303 RT) (where T is temperature in Kelvin and qads heat of adsorption) and Figure 17 plotted against log(θ/1-θ) versus 1/T which produced straight lines. Heat adsorption values found to be negative with nanocoating and filler compounds so the formation of composite barrier is a chemical process. These compounds were adhered with base material by chemical bonding.
Free energies of both compounds give information that coating is an exothermic process. It is clear by data calculated for octahydrodibenzo[a,d][8]annulene-5,12-dioxime and ZnS filler with the help of equation, ΔG =-2.303RT log (33.3K) (where R is universal gas constant, T be temperature and K corrosion rate) and their values were written in Table 2 and plotted in Figure 20. Both compounds were produced a negative heat of adsorption which indicated that they formed chemical bonding during nanocoating process.
Figure 20:ΔG Vs T for (θ) for nanocoating of NC (5) & ZnS filler on polybutadiene-coating mild steel
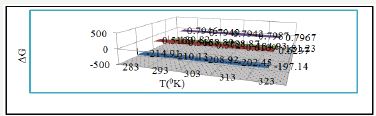
Figure 21:ΔE (mV) Vs I (mA/cm2) for nanocoating of NC(1) & filler ZnS on polybutadiene-coated mild steel.
Enthalpy and entropy are very important thermal parameters which give information about nanocoating and filler compounds bonding nature with base materials. The values of enthalpy and entropy for octahydrodibenzo[a,d][8]annulene-5,12-dioxime and ZnS filler were calculated by transition state equation, K=RT/Nh log (ΔS# /R) X log (-ΔH #/RT), (where N is Avogadro’s constant, h is Planck’s constant, ΔS# is the change of entropy activation and ΔH# is the change of enthalpy activation) and Figure 21 and their values were recorded in Table 2. The results of enthalpy and entropy were shown that both compounds were adsorbed with the base materials by chemical bonding. Such coating is an exothermic process. The negative entropy indicated that nanocoating and filler compounds accommodated on the surface of polybutadienecoated mild steel in an ordered matrix. Figure 21 plotted between free energy (ΔG) and temperatures for surface coverage area (θ) occupied by octahydrodibenzo[a,d][8]annulene-5,12-dioxime and ZnS filler that confined free energy decreased when temperatures increased but at this moment surface coverage area was enhanced.
Table 2: Thermal parameters of nanocoating compound octahydrodibenzo [a,d][8] annulene-5, 12-dioxime and ZnS filler coated on polybutadiene-coated mild steel in marine water.
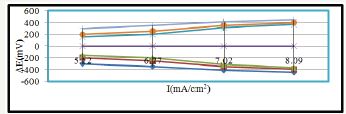
The corrosion potential, corrosion current and corrosion current density of polybutadiene-coated mild steel, nanocoated octahydrodibenzo[a,d][8]annulene and ZnS filler were calculated by equation, ΔE/ΔI = βa βc /2.303 Icorr (βa +βc ) (where ΔE/ΔI is the slope which linear polarization resistance (Rp , βa and βc are anodic and cathodic Tafel slope respectively and Icorr is the corrosion current density in mA/cm2) and Tafel plot between electrode potential (ΔE) versus current density (I) and their values were mentioned in (Table 3). It was observed that electrode potential and corrosion current density were high with polybutadiene whereas anodic current density increased and cathodic density current reduced. But octahydrodibenzo[a,d][8]annulene-5,12- dioxime and ZnS filler reduced electrode potential and corrosion current density. Tafel plot of Figure 22 and the results of Table 3 indicated that nanocoating and filler compounds minimized anodic current and maximized cathodic current. These results confirmed that nanocoaing and filler compounds developed a strong barrier on the polybutadiene-coated mild steel which neutralized the attack of saline water and carbonic acid.
Figure 22:XRD of octahydrodibenzo[a,d][8]annulene-5,12- dihydrazone.

Table 3: Potentiostat polarization of octahydrodibenzo[a,d][8] annulene-5,12-dioxime and ZnS nanocoated on polybutadienecoated mild steel in marine water
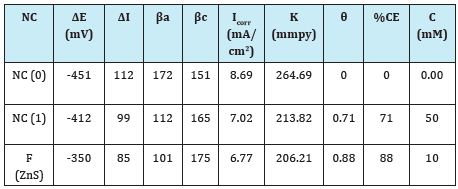
The corrosion current was obtained by above equation and Tafel graph of Figure 22 for polybutadiene-coated mild steel, nanocoated octahydrodibenzo [a,d][8]annulene and ZnS filler and these values were put in equation, C. R (mmpy) = 0.1288 I (mA/ cm2) × Eq .Wt (g) / ρ (g/cm3) (where I is the corrosion current density ρ is specimen density and Eq. Wt is specimen equivalent weight) and their values were recorded in Table 3. It was noticed that corrosion rate increased with polybutadiene-coated mild steel and these values were reduced with nanocoating and filler compounds. The results of corrosion rate measured by weight loss experiment are confirmed the results of potentiostat. Potentiostat polarization of octahydrodibenzo[a,d][8]annulene-5,12-dioxime and ZnS nanocoated on polybutadiene-coated mild steel in marine water (Table 3).
Conclusion
It is very difficult to control corrosion of marine water. Polybutadiene-coated mild steel uses in marine water for different works but this material is face corrosion problem. In this research, it is tried to check corrosion of polybutadiene mild steel by the application of nanocoating compound octahydrodibenzo[a,d][8] annulene-5,12-dioxime and filler ZnS. The corrosion activities of polybutadiene was studied at 283 0K, 293 0K, 303 0K, 313 0K and 323 0K temperatures and the concentration of nanocoating and filler compounds were taken in 50mM and 10mM. The results of surface coverage areas and coating efficiencies of nanocoating and filler compounds were indicated these compounds had more coverage capability. Nanocoating and filler compounds results of activation energy, heat of adsorption, free energy, enthalpy and entropy were shown that these compounds were attached with base material by chemical bonding. They can form composite thin film barrier which is passive in corrosive environment. Filler material blocks the porosities of nanocoating compounds and stop osmosis or diffusion process of corrosive agents.
Acknowledgement
Author is very thankful to finical agency UGC-New Delhi, India providing research grant. I also give thanks my research Mr. Manjay Kumar Thakur for his laboratory work and data calculation.
References
- Bhadra S, Singh NK and Khastgir D (2011) Polyaniline based anticorrosive and anti-molding coating. J Chem Eng Mater Sci 2(1): 1-11.
- Szabo T, Molnar-Nagy L, Telegdi J (2011) Self-healing microcapsules and slow release microspheres in paints, Progress in Organic Coatings 72(1- 2): 52-57.
- Videla H, Herrera LK (2009) Understanding microbial inhibition of corrosion. A comprehensive overview. Electro chem Acta 63(7): 896- 900.
- Wen NT, Lin CS, Bai CY, Ger MD (2008) Structures and characteristics of Cr (III) based conversion coatings on electrogalvanized steels. Surf Coat Technol 203: 317-323.
- Boerio FJ, Shah P (2005) Adhesion of injection molded PVC to steel substrates. J of Adhesion 81(6): 645-675.
- Deveci H, Ahmetti G, Ersoz M (2012) Modified styrenes: Corrosion physico-mechanical and thermal properties evaluation. Prog Org Coat 73: 1-7.
- Genzer J (2005) Templating Surfaces with Gradient Assemblies. J of Adhesion 81(3-4): 417-435.
- Leon-Silva U, Nicho ME (2010) Poly(3-octylthiophhene) and polystyrene blends thermally treated as coating for corrosion protection of stainless steel 304. J Solid State Electrochem 14(8): 1487-1497.
- Baier RE (2006) Surface behaviour of biomaterials: the theta surface for biocompatibility. J Mater Sci Mater Med 17(11): 1057-1062.
- Rao BVA, Iqbal MY, Sreehar B (2010) Electrochemical and surface analytical studies of the self assembled monolayer of 5-methoxy- 2-(octadeclthiol) benzimidazole in corrosion protection of copper. Electrochim Acta 55(3): 620-631.
- Liu XY, Ma HY, Hou MZ (2009) Self-assembled monolayers of stearic imidazoline on copper electrodes detected using electro chemical measurement, XPS, molecular simulation and FTIR. Chinese Sci Bull 54: 374-381.
- Liao QQ, Yue ZW, Zhou Q (2009) Corrosion inhibition effect of selfassembled monolayers of ammonium pyrrolidine dithiocarbamate on copper. Acta Phys Chin Sin 25(8): 1655-1661.
- Zhang DQ, He XM, Kim GS (2009) Arginine self-assembled monolayers against copper corrosion and synergetic effect of iodide ion. J Appl Electrochem 39(8): 1193-1198.
- Ghareba GS, Omanovic S (2010) Interaction of 12-aminododecanoic acid with a carbon steel surface: Towards the development of ‘green’ corrosion inhibitors. Corrosion Sci 52(6): 2104-2113.
- Sahoo RR, Biswas SK (2009) Frictional response of fatty acids on steel. J Colloid Interf Sci 333(2): 707-718.
- Raman R, Gawalt ES (2007) Selfassembled monolayers of alkanoic acid on the native oxide surface of SS316L by solution deposition. Langmuir 23(5): 2284-2288.
- Li DG, Chen SH, Zhao SY (2006) The corrosion Inhibition of the selfassembled Au and Ag nanoparticles films on the surface of copper. Colloid Surface 273: 16-23.
- Cristiani P (2005) Solutions fouling in power station condensers. Appl Therm Eng 25(16): 2630-2640.
- Bibber JW (2009) Chromium free conversion coating for zinc and its alloys. Journal of Applied Surface Finishing 2(4): 273-275.
- Singh RK (2016) Corrosion protection of transport vehicles by nanocoating of decahydrobenzo [8]annulene-5,10-dihydrazone and SiC filler in H2O(moist), CO2, SO2 environments and weather change, Journal of Metallurgy and Materials Science 58: 167-179.
- Singh RK (2017) Corrosion protection of transport vehicles by nanocoating of decahydrobenzo [8]annulene-5,10-dihydrazone in corrosive environments and weather change. J Powder Metall Min 1: 2-8.
- Singh RK (2017) Atmospheric corrosion protection of epoxy-coated stainless steel by nanocoating of decahydrobenzo [8]annulene-5,10- disemecarbazone and TiN filler. International J of NME 2(4): 17-32.
© 2017 Rajesh Kumar Singh, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






