- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Catheter Cryoablation of Ventricular Ectopy Originating from His Region
Andrea Rossi*, Luca Panchetti, Umberto Startari, Bruno Formichi, Gianluca Mirizzi and Marcello Piacenti
Invasive Cardiology Division, Arrhythmology Unit, Italy
*Corresponding author: Andrea Rossi, Invasive Cardiology Division, Arrhythmology Unit, Fondazione G. Monasterio CNR-Regione Toscana
Submission: April 28, 2018;Published: May 08, 2018

ISSN 2578-0204Volume2 Issue1
Abstract
Catheter ablation of premature ventricular complexes (PVCs) is an effective treatment when disabling symptoms or ectopy-induced cardiomyopathy are present in patients refractory to pharmacological therapy [1,2]. When PVCs originate close to His bundle, radiofrequency ablation is burdened by unacceptable risk of conduction pathways damage or atrio-ventricular block [3]. Here we report a case of a patient with highly symptomatic ventricular ectopy originating close to His bundle undergoing successfull cathetercryoablation.
Keywords:Premature ventricular complexes; Bundle of his; Catheter ablation; Cryothermal ablation
Case Presentation
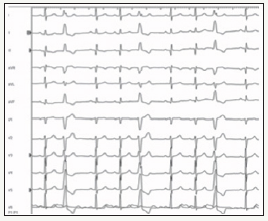
A 47-year-old man with symptomatic frequent PVCs, refractory to pharmacological therapy with betablockers and flecainide in association, was admitted for catheter ablation. A 12-lead electrocardiogram (ECG) of PVCs showed a monophasic R wave in lead 1 and aVL with precordial transition in V4 (Figure 1). Ambulatory ECG recording showed frequent monomorphic PVCs (60,230 ectopic beats; 35% of the total beats).
Figure 1:12-lead ECG shows frequent clinical PVCs.
After informed consent was obtained, a cardiac electrophysiological study was performed under conscious sedation. Baseline ECG showed normal sinus rhythm with frequent monomorphic PVCs with the same configuration as clinical morphology. Diagnostic catheters was advanced for reference in coronary sinus and His-region.Electrophysiological studywith programmed ventricular stimulation did not inducesustained ventricular tachycardias in basal conditions orduring isoproterenol infusion. Bipolar ventricular signal recorded from His-catheter advanced PVC onset by 20msec (Figure 2). Activation mapping was performed using a 8-Fr open irrigated catheter with contact force sensor with an electroanatomic mapping system. Right ventricular (RV) activation mapping focused on PVCs beats showed an early focal activation in the region on the inflow tract. The target site was recognizedbetween “valvular” His-potential region and right bundle branch origin. At the mapping site local bipolar signal recorded from mapping catheter advanced PVC onset by 47 msec with unipolar QS pattern (Figure 2). Pacemapping conducted from ablator tip demonstrated optimal concordance (12/12 leads)in respect tothe ectopic beat (Figure 3). At this site an RF application was performed with low energy (20 W with a target temperature of 45°C) with complete disappearence of ectopic beats but rapid right bundle block creation. Application was then stopped. After 2 minutes conduction damage was resolved but ectopic activity recurred. In order to avoid persistent conduction damage, a 7-Fr cryoablator catheter (Freezor, Medtronic) was advanced for mapping and ablation. An accurate cryomapping was conducted in the target site up to -35°C. Cryoablation up to -80°C were performed for 6 minutes under continous check for AH and HV prolongationand right bundle block occurrence. Afterwards, the successfull result persisted during 30 minute observation (Figure 4). The patientwasdischarged without complications after two days from the procedure. Ambulatory ECG monitoring showed a PVCs burden of 4% and 2% at 2 months and 1 year of followup, respectively. No conduction abnormality was noted during monitorings. The patient, in the absence of antiarrhythmic drugs, remained free from symptoms during afollow-up period of one year.
Figure 2:Recordings on ablation site of ablation catheter (ABL) and His catheter (His). Local ventricular activation recorded on His catheter (His p) advanced PVC onset by 20msec. Local ventricular activation of ablation catheter is earlier than onset of PVC by 47ms. Note the His signal (H) recorded at the ABLp in the sinus beat.
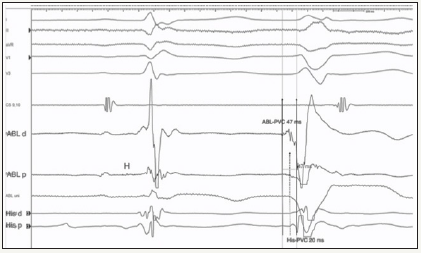
Figure 3:Activation mapping of ventricular ectopy (A). Red dots shows location of “valvular” His bundle. There is a perfect match between pacemap from the ablation site (B) respect to spontaneous ectopic beat (C).
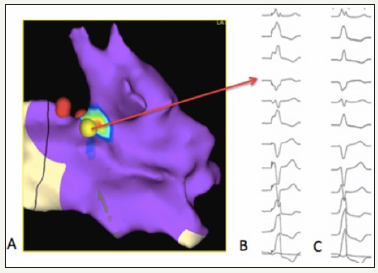
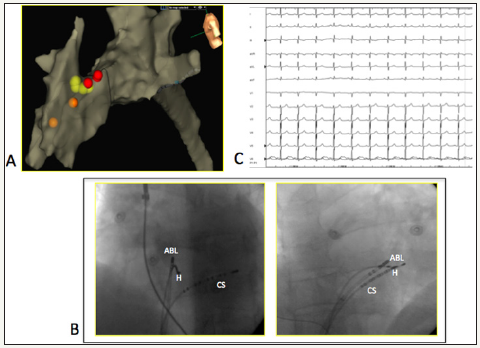
Figure 4:Electro anatomical mapping of right heart (A), modified left-lateral view. Red dots are location of “valvular” His. Orange dots are mapping points of right bundle branch signals. Yellow dots are cryoenergy applications in the target site (angiographic LAO and RAO views, image B). Image C is the electrocardiographic final result after successfull cryoablation.
Discussion
We reported a successfull catheter cryoablation of PVCs originating from the hisian region without AV conduction damage. Electrocardiographic analysis of ectopic beatsshows vertical axis with large positive notched R wave in D1 and pattern QS in AVR with rSr’ on AVL; precordial transition was in V4 with QS pattern in V1 and large R wave in V5-V6. Based on the electrocardiographic features we presumed the focus to be located near the His-region. Activation mapping indicated the exit-site of ectopics at the proximal RV inflow tract and the presence of His signal recorded on the mapping catheter indicate that the ablation site was near the main penetrating AV conduction bundle. Given the electrophysiological features found on the ablation target site (bipolar and unipolar electrograms, pacemapping and effect of energy delivery), we decided not to perform an additional mapping on the left side of the interventricular septum or non-coronary aortic cusp.
It is intersting to note that in our report irrigated RF delivery permitted to suppress ventricular ectopy but with transient right bundle block creation; after 2 minutes from RF ablation attempt conduction damage was resolved but ectopic activity recurred. At the target site cryothermal energy applicationled to ectopy suppression without right bundle block creation. While a more superficial lesion together with optimal catheter stability obtained during cryoablation may allow to suppress ventricular ectopy and avoid damage of deeper penetrating structures, irrigate RF energy could have determined a deeper lesion leading to right bundle branch block.
Safety and feasibility ofparahisian focal cryoablation for ventricular arrhythmias is well reported in the literature. Several papers described cases of successfull ablation procedures using irrigated and non-irrigated RF energy [4,5]. De Biase et al. [5] reported the largest population of 7 cases of ventricular arrhythmias originating from His region treated with successfull cryoablation. No complication or failure were mentioned. The average distance from ablation site to critical conduction structures were 4 mm about. In our opinion this case report may be of interest for specialists who are aware of highly unwanted complications during ablation in parahisian region. Furthermore this report shows the importance of changing approach and the ablation setting in difficult situations. As a matter of fact, after transient right bundle block creation we discontinued RF delivery and converted our procedure to the cryothermal setting.
Conclusion
In conclusion, we performed a successfull catheter cryoablation of a PVC from the His region under activation and electroanatomical mapping guidance. Careful mapping, early detection of AV conduction damage and cryothermal energy availability are essential in dealing with ablation procedures at the parahisian region.
Ethical Standards
This study complies with the Declaration of Helsinki and our Institutional Ethics Committee approved the study protocol. As mentioned in the text, informed consent from our patient was obtained before the invasive procedure.
References
- Zhu DW, Maloney JD, Simmons TW, Nitta J, Fitzgerald DM, et al. (1995) Radiofrequency catheter ablation for management of symptomatic ventricular ectopic activity. J Am Coll Cardiol 26(4): 843-849.
- Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, et al. (2007) Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm 4(7): 863-867.
- Gondo T, Yoshida T, Inage T, Takeuchi T, Fukuda Y, et al. (2012) How to avoid development of av block during rf ablation: anatomical and electrophysiological analyses at the time of AV node ablation. Pacing Clin Electrophysiol 35(7): 787-793.
- Kim J, Kim JS, Park YH, Kim JH, Chun KJ (2011) Catheter ablation of parahisian premature ventricular complex. Korean Circ J 41(12): 766- 769.
- Di Biase L, Al Ahamad A, Santangeli P, Hsia HH, Sanchez J, et al. (2011) Safety and outcomes of cryoablation for ventricular tachyarrhythmias: results from a multi-center experience. Heart Rhythm 8(7): 968-974.
© 2018 Andrea Rossi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






