- Submissions

Full Text
Open Journal of Cardiology & Heart Diseases
Commentary: The number of Electrons in the Nernst Equation: Energetic Considerations
Mark IM Noble*
University of Aberdeen, UK
*Corresponding author: Mark IM Noble, University of Aberdeen, Scotland, UK
Submission: August 03, 2017; Published: November 07, 2017

ISSN: 2578-0204 Volume1 Issue1
Introduction
The Nernst equation is written: Ecell = E0Cell − ( RT/nF) InQ
Ecell=cell potential under nonstandard conditions (V)
E0Cell=cell potential under standard conditions, in which there is a negative trans-membrane potential, i.e., a net excess of intracellular negative charges.
R = gas constant, which is 8.31 (volt-coulomb)/(mol-K)
T = temperature (K)
n=number of moles of electrons exchanged in the electrochemical reaction (mol)
In summary, recent evidence suggests that local thrombolysis can be effective and safe, expecially in high risk pulmonary embolism patients with high risk of consequent hemorrage. Postoperative cardiac patients seem to meet those criteria. Obviously further research is essential before we start using routinely this intriguing and promising technique.
F = Faraday’s constant, 96500 coulombs/mol
Q=Reaction quotient, which is the equilibrium expression with initial concentrations rather than equilibrium concentrations
Note that the source of electrons contributing to “n” is not specified. “The present theory is that n equals the number of electrons lost to the cell during depolarisation”,
For the equation to function there is a requirement for electrons.
The role of potassium ions under standard conditions
The Nernst equation is also sometimes written:
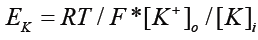
[K+]o = extracellullar potassium ion concentration
[K+]i = intracellular potassium ion concentrantion
There is no indication in this version of electrons. It is well known that increasing [K+]o depolarises cells; one can arrest the heart that way. This is easily explained by excess positively charged particles reacting with the net negatively charged cell.
[K+]i is high, [K+]o is low. Extracellular sodium ion concentration ([Na+]o) is high. Intracellular sodium ion concentration ([Na+]i) is low
The advocates of the idea that the net negative charge of living cells (and therefore negative trans-membrane potential) is the result of the K+ concentration difference between the intracellular and extracellular compartments, maintain that this charge difference is the result of the actions of the membrane Na+/ K+ ATPase, the “sodium pump” (Figure 1).
Figure 1: Typical diagram for conventional teaching to undergraduates, showing a cell with negative transmembrane potential dependent on the action of the sodium pump - the “membrane pump theory”.
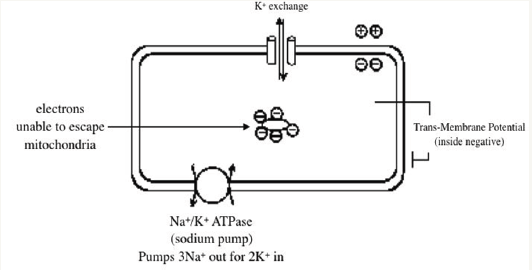
The sodium pump consumes energy in the form of adenosine triphosphate (ATP) to maintain a low intracellular sodium. An example in the case of Purkinje cells is that, if the intracellular Na+ concentration is increased by doubling the frequency of activations, the pump is activated to restore the original concentrations over a period of about 10 seconds [1]. As a result there is net loss of positive charges and a small increase in negativity of the transmembrane potential [1].
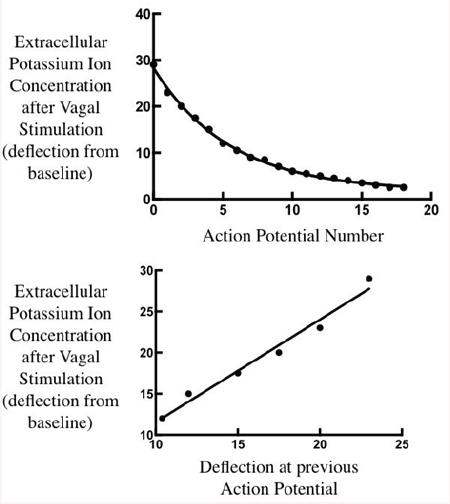
The advocates of the membrane pump theory think that this same pump produces the high [K+]i, but it is not possible for the same enzyme to be drive by both [Na+]i and [K+]o. This is shown by analysis of the experiments of Kronhaus et al. [2] using K+ sensitive electrodes, who raised [K+]o in sino-atrial node using vagal stimulation. The high [K+]o declines exponentially, so that by plotting [K+]o of each activation against that of the previous activation, one obtains a straight line, the slope of which expresses the recirculation of K+ within the extracellular space (Figure 2). This turns out to be 100%. There is no extra entry of K+ into the cells in spite of sodium pump cycling. Excess extracellular K+ does not activate the sodium pump. Thus the high [K+]i, compared to [K+o] is not produced by the Na+/K+ ATPase.
Figure 2: Upper panel: Exponential decay of extracellular potassium ion concentration. Values taken from tracing published by Kronhaus et al. [2]. Lower panel: Values plotted against that of the previous value during the early steep part of the decay. The slope of the line is close to 1.0, indicatng 100% recirculation of potassium ions within the extracellular compartment and no acceleration of Na+/ K+ ATPase.
In conformity with the small positive effect on transmembrane potential of accelerating the cell membrane Na+/K+ ATPase [1] is the small negative effect on trans-membrane potential of inhibition of the sodium pump with ouabain [3].
The role of mitochondria
The membrane pump theory has also been criticised on the basis that there is insufficient energy available to achieve the high [K+]i using the membrane pump [4,5]. The energy for ATPase based functions is supplied as ATP produced by mitochondria, which generate electrons to provide the electromotive force required to drive ATP synthase. Another “established” idea is that that electrons generated by mitochondria are unavailable for any process taking place in the main cellular cytoplasmic gel. (The idea that electrons cannot travel in cells because they cannot travel through electrolyte is manifestly wrong. If it were true, magnetic resonance imaging could not exist).
The theorists of these ideas are therefore unable to consider the more simple explanation for the net negative charge of the cytoplasm in that some electrons, from the electron flow induced by oxydative phosphorylation, cross the mitochondrial membrane which has a variable electrical conductance. Mitchell’s hypothesis [6] proposed coupling between electron transport and phosphylation (breaking this coupling inhibits ATP production) [7].
The conventional theory to account for the negative cellular transmembrane potential (Figure 3) involves converting substrate energy to electrical energy, using electrons to generate chemical energy in the form of ATP, dissipation of that energy to transfer potassium ions from the extracellular to the intracellular space and generating electrons for the Nernst equation. In an alternative scheme is illustrated in Figure 4, in which there is an inward current from the cell cytoplasm (at -60 - 90mV) to the mitochondria (at 180-220mV [8], as long as the mitochondrial membrane is not an absolute insulator; it is actually known to have finite electrical conductance. Such an inward current is carried by electrons flowing from the mitochondia to the cell cytoplasm.
Figure 3: Conventional scheme for establishment of inner to outer potassium ion gradient and negative transmembrane potential.
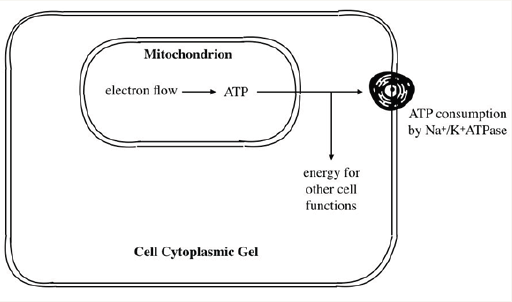
Figure 4: Alternative scheme in which there is an inward current from the cell cytoplasm (at -60 - 90mV) to the mitochondria (at 180-220mV) as long as the mitochondrial membrane is not an absolute insulator; it is actually known to have finite electrical conductance. Such an inward current is carried by electrons from the mitochondia to the cell cytoplasm.
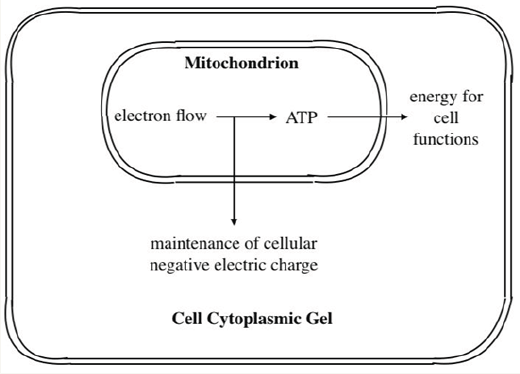
One now evokes the Law of Natural Selection and asks, “Would nature evolve extra energy consumption to generate cellular negative charge using the energy expensive Na+/K+ ATPase, when electrons are already available from the mitochondrial electron chain?” It now remains to postulate the mechanism of the high intracellular K+ concentration, most of which be clearly cannot be achieved by the Na+/K+ ATPase (Figure 2). Intracellular K+ are aligned along the protein matrix which confers the gel state of the cytoplasm, perhaps by association with ß- and γ- carboxyl groups of intracellular proteins [9]. The association of K+ with the protein filaments is the basis of the electrostatic field synthesis of muscle cells [10]. This theory is in accordance with the fact that the intracellular compartment is not liquid, as is assumed by the membrane pump theory. The high K+ content of the protoplasm is an essential feature of the gel state of living cells.
The problem of the mechanism of repolarisation
The Nernst equation as stated in the introduction describes the unstable state. A comment may be made here in that, in electrically excitable cells, the unstable state is caused by depolarisation, which triggers the cells active functions. Conventional thought states that repolarisation (to re-attain the stable state) is carried by an outward flow of K+, because repolarisation current is K+ dependent. (Actually all intracellular electrical events are K+ dependent according to the theory associating K+ with the protein matrix and providing the electrical field synthesis that provides intracellular electrical conductivity). However, the analysis in Figure 2 shows that there is no K+ pump to return the K+ to the intracellular compartment, and therefore (especially in a regularly cycling group of cells such as cardiac cells) there can be no steady state for K+. Therefore a different mechanism of repolarisation must be postulated and follows from the sheme illustrated in Figure 4.
If we take the extreme example of the cardiac ventricular cell, depolarisation involves a change in cellular trans-membrane potential from -80 to +40mV, and hence a large increase in the mitochondrial trans-membrane potential and inward current. The inevitable negative feedback mechanism of the mitochondrion will accelerate the rate of oxidative phosphorylation, ATP production for increased energy requirements of the activated cell and increased electron transfer to the cytoplasm, thus achieving repolarisation (restoration of resting negative charge), without the need for K+ cellular depletion and unecessary energy consumption to return K+ to the intracellular compartment.
References
- Boyett MR, Hart G, Levi AJ, Roberts A (1987) Effects of repetetive activity on developed force and intracellular sodium in isolated sheep and dog purkinje fibres. J Physiol 388: 295-322.
- Kronhaus KD, Spear JF, Neil-Moore E (1978) Sinus node extracellular potassium transients following vagal stimulation. Nature 275(5678): 322-324
- Miura DS, Rosen MR (1978) The effects of ouabain on the transmembrane potential and intracellular activity of canine cardiac Purkinje fibers. Circ Res 42(3): 333-338
- Ling GN (1997) Debunking the alleged resurrection of the sodium pump hypothesis. Physiol Chem Phys Med NMR 29(2): 123-198.
- Ling GN (2007) History of the membrane (pump) theory of the living cell from its beginning in mid-19th century to its diproof 45 years ago - though still taught worldwide today as established truth. Physiol Chem Phys Med NMR 39(1): 1-67.
- Mitchell P (1966) Chemiosmotic coupling in oxidative phosphorylation and photosynthetic phosphorylation. Biol Rev 41(3): 445-501
- Terada H (1990) Incouplers of oxidative phosphylation. Environ Health Perpesct 87: 213-218.
- Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA (2011) Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniquies 50: 98-115.
- Ling GN (2005) An updated and further developed theory and evidence for close-contact, one-on-one association of nearly all cell K+ with ß- and γ- carboxyl groups of intracellular proteins. Physiol Chem Phys Med NMR 37:1-63.
- Iwazumi T (1970) A new field theory of muscle contraction. PhD Thesis, University of Philadelphia, USA.
© 2017 Mark IM Noble. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






