- Submissions

Full Text
Open Access Research in Anatomy
Comparative Assessment of Zinc Sulphate and Essentiale Forte® in Managing Biochemical Alterations of Carbon Tetrachloride-Induced Hepatotoxicity on Adult Wistar Rats
Arayombo Babatunde E*, Adewole Olarinde S, Adelodun Taiwo S, Saka Olusola S and Ronald Bejide A
Department of Anatomy and Cell Biology, Obafemi Awolowo University, Nigeria
*Corresponding author: Arayombo Babatunde E, Department of Anatomy and Cell Biology, Faculty of Basic Medical Sciences, Obafemi Awolowo University, Ile-Ife, Nigeria
Submission: January 29, 2018;Published: May 10, 2018

ISSN: 2577-1922
Volume2 Issue1
Abstract
Objective: This study was to assess and compare the effect of Zinc Sulphate and Essential Forte® on the biochemistry of carbon tetrachloride (CCl4) induced hepatotoxicity in adult Wistar rats.
Material and method: Twenty five adult Wistar rats, weighing between 150 g and 170 g, were used for the study. They were housed in plastic cages fed on standard laboratory rat pellets and given water ad libitum. The animals were divided into five groups A, B, C, D and E (n=5). Group (A) received 0.7ml/kg of olive oil orally. Groups B, C, D and E were administered CCl4 (0.7ml/kg orally) for 1 week in 1:1 dilution with olive oil. After CCl4 administration Group C was treated with Essentiale Forte® (4.5mg/kg bw orally) for four weeks. Group D was treated with Zinc Sulphate (7mg/kg bworally) daily for four weeks. Group E received Zinc Sulphate (7mg/kg bw orally) and Essentiale Forte® (4.5mg/kg bw orally) for a period of 4 weeks, while group B was left untreated. Animals were left for another one week and afterwards sacrificed under ether anaesthesia. Blood samples were obtained via cardiac puncture and centrifuged to obtain serum. Markers of liver function such as, Aspartate Amino Transferase (AST), Alanine Amino Transferase (ALT) as well as Alkaline Phosphatase (ALP) were estimated in the serum using enzyme colorimeter assay kit (Randox). Data obtained were analyzed by one way ANOVA, then Student Newman-Keuls (SNK) test for multiple comparisons.
Results: Treatment with Zn and or Essentiale Forte® restored the hepatic function as the biochemical analysis with Mean±SEM showed significant reduction in the levels of markers of liver function and protected the liver tissue from fatty and degenerative changes.
Conclusion: This study showed that combination of Essential Forte® and Zinc supplement offered better ameliorative effect on the markers of liver function of Wistar rats following CCl4 induced-hepatotoxicity compared with separate administration of either Essential Forte® or Zinc supplement.
Introduction
Most routine tests reflect liver damage the tests of function are those which reflect synthetic capacity for instance albumin and prothrombin time, liver function tests may be grossly deranged when function is normal and may be normal when function is grossly deranged for it has enormous functional reserves, such that early liver impairment is clinically marked and the progression of the deranged liver function makes the condition life threatening [1]. Morphologically, liver responds to injurious events in 5 different ways, irrespective of the cause viz; Degeneration and intracellular accumulation, Necrosis and Apoptosis, Inflammation, Regeneration, Fibrosis [1].
CCl4 is a colourless liquid, ether-like in odour with a density of l.6gcm-3 melting point is 22.9OC, boiling point is 76.7 OC and soluble in water at 0.08g/100ml (25 OC). It is also soluble in alcohol, ether, chloroform, benzene, naptha and carbon sulfide. The vapour pressure is 11.94kPa at 20 OC and refractive index of 1.5, it has crystal structure with tetrahedral shape. It is not flammable, its auto-ignition tempt is 982 OC and LD50 is 2350mg/kg. International programme on chemical safety (IPCS) (1999) It has been reported to produce free radical which affects the cellular permeability of the liver cells leading to altered level of serum biochemistry and liver enzymes [2]. In time series, it was found to have an atmospheric life time of 85 years and liver damage inflicted by CCl4 has lethal consequences.
For so many years, liver disease remains one of the major health concern despite discovery and development of new drugs, morbidity and mortality accompanying hepatic pathology is still clinically significant [3]. Chronic exposure of liver to CCl4, has been researched with consequent spectrum of chronic liver disease, such as fatty liver, hepatitis, liver cirrhosis and hepatoma [3]. Liver damage can occur after 24hrs of exposure to CCl4 and in serious cases this can result in hepatitis, ascites, hemorrhages, hepatic coma and even death. International programme on chemical safety (IPCS) [4].
Essential phospholipid has since been used in the cases and managements of chronic liver disease. It is a 300mg hard gel capsule that contains De-oiled enriched phospholipids from neutral soya beans. Its mechanism of work by phospholipids replacement and regeneration in the liver cell membranes, which have been damaged by various means especially through liver toxin. The pharmaceutical excepients are chiefly ethanol, hard fat, Hydrogenated, Castor oil, Ethyl vanillin, 4-methoxylphenylethanone, Alpha-tocopherol, Gelatin, Colouring agents, Sodium laurylsulfate, Purified water. Sonafi avens is pharmaceutical.
Zinc is known as an essential trace element necessary for protein metabolism, as well as membrane integrity and also involved in the structure and function of numerous metalloenzymes. It has important functions in skin and connective tissue, metabolism as well as in wound healing [5]. It exerts its antioxidant effects indirectly by maintaining membrane structures, involving in the structure of SOD, increasing the metallothionein concentrations and, competing with redox reactive metals, iron and cuprous for critical binding sites [6]. It is shown that hepatic and serum zinc levels of patients in liver disease decreased depending on the degree of liver damage [7].
Material and Method
Animal care and management
Twenty five adult Wistar rats, weighing between 150g and 170g (6-10 weeks old) obtained from Animal Holding of International Institute of Tropical Agriculture Ibadan Oyo State were used for the research. The animals were housed in plastic cages in a clean environment of 12 hours day/light cycle, at room temperature, in the animal holding of the Department of Anatomy and Cell Biology. They were fed on standard laboratory rat pellets and have free access to water. Ethical clearance for the study was obtained from Health Research Ethical Committee (HREC), Institute of Public Health (IPH) Obafemi Awolowo University(OAU) Ile-Ife. The animals were given humane care according to the guidelines of HREC, IPHOAU.
Preparation of the chemicals/drugs
2.5 litres of CCl4 was obtained from the central research laboratory of Obafemi Awolowo University, Olive Oil, Zinc Sulphate (ZnSO4) tablets and Essentiale Forte® capsules were of the best grade commercially available. 60ml of CCl4 was diluted with 60ml of olive Oil in 1:1 equivalent, and this was administered at a dose of 0.7ml/kg p.o. 3 tablets of ZnSO4 was dissolved in 60ml of water and this was administered at a dose of 7ml/kg, Essentiale Forte® capsule was prepared by dissolving a capsule in 60ml of water, and was administered at a dose of 4.5mg/kg all being freshly prepared on each day of administration.
Animal treatment
The rats were divided into five groups A, B, C, D and E of five rats each (n=5). Group (A) normal control, received oral administration of olive oil only. Group B negative control received daily administration of CCl4 (0.7ml/kg p.o) for 1 week in 1:1 dilution with olive oil without treatment. Groups C test group I received Essentiale Forte® 4.5mg/kg/day for four weeks after the administration of CCl4. Group D test group II received Zinc Sulphate® (7mg/kg/day p.o) daily for four weeks after the administration of CCl4. Group E test group III received Zinc Sulphate® 7mg/kg/day for four weeks and Essentiale Forte (4.5mg/kg/day p.o) for a period of 4 weeks concurrently, after CCl4 administration. All administrations were via oral for four weeks (Essentiale Forte® and Zinc Sulphate®) while CCl4 was for one week.
Animal sacrifice and sample collection
At the end of the experimental procedure the animals were sacrificed after one week of recovery period. Animals were euthanized by ether anaesthesia a midline incision was made along the anterior abdominal wall. The blood was taken by cardiac puncture, the abdomen of the sacrificed animal will be cut open quickly and the liver per fused with isotonic saline, excised, blotted dry, weighed, and divided into samples. Liver tissues were excised from sacrificed animals, weighed, and thin liver slices were cut, fixed and were sequentially embedded in paraffin wax blocks. Five μ thick sections were cut, and stained with Hematoxylin-Eosin (HE) and Masson’s Trichrome for conventional morphological evaluation, then examined under light microscope (Olympus). The degree of hepatic necrosis and fibrosis were determined by a semi-quantitative method [8]. Some portions of the liver tissues were homogenized for biochemical assay (ALT, AST, ALP) and the rest fixed in 10% formal saline for subsequent routine histological procedures.
Serum and homogenate Alanine, Aspartate Aminotransferases and Alkaline Phosphatase (ALT, AST and ALP) Whole blood was centrifuged at 4700rpm for 10min at 4 OC and ALT, AST and ALP were determined spectrophotometrically with an automatic analyzer (Cobas Mira; Roche, Rotkreuz, Switzerland) using commercially available kits (Randox Diagnostics). Their activities were expressed as an international unit (IU/L). ALT, AST and ALP activities in liver homogenates were also determined using a quantitative, colorimetric end-point Randox assay kit (Procedure No. 104, Sigma Diagnostics, St. Louis, MO) that used α-ketoglutaric acid as the substrate and that detected production of pyruvic acid. Results were expressed as international units (IU).
Statistical Analysis
One way ANOVA was used to analyze the data, followed by Student Newman-Keuls (SNK) test for multiple comparisons. Graph Pad Prism 5 (Version 5.03 Graph Pad Inc.) was the statistical package used for data analysis. Significant difference was set at p<0.05.
Results
Hepatic enzyme levels in the Control and test groups (Table 1 and Figure (1-5)).
Figure 1 The graph showing the ALP levels of rats across the group after treatment.
Results presented as mean±SEM(n=5); α: Significantly different from normal control at p<0.05; β: Significantly different from toxic control at p<0.05; δ: Significantly different from C,D and E at p<0.05
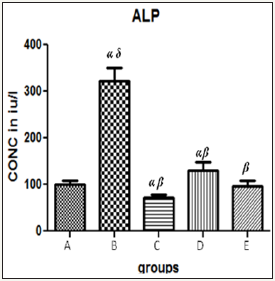
Figure 2 The graph showing AST levels of the rats across the group after treatment.
Results presented as mean±SEM(n=5); α: Significantly different from normal control at p<0.05; β: Significantly different from toxic control at p<0.05; δ: Significantly different from C,D and E at p<0.05
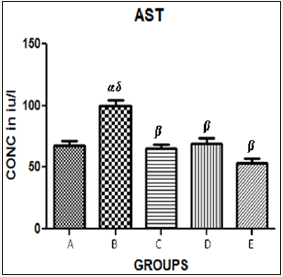
Table 1 Hepatic enzyme levels in the control and test groups.
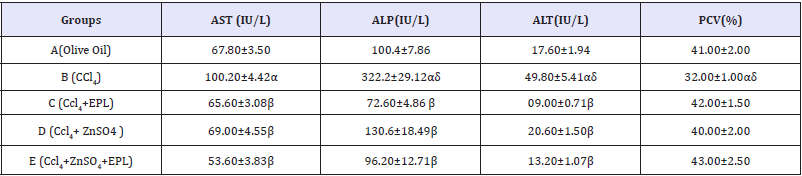
Results presented as mean±SEM(n=5); α: Significantly different from normal control at p<0.05; β: Significantly different from toxic control at p<0.05; δ: Significantly different from C,D and E at p<0.05; EPL: Essentiale forte®; CCl4: Carbon tetrachloride; ZnSO4: Zinc Sulphate
Figure 3 The graph showing ALT levels of the rats across the group after treatment.
Results presented as mean±SEM(n=5); α: Significantly different from normal control at p<0.05; β: Significantly different from toxic control at p<0.05; δ: Significantly different from C,D and E at p<0.05
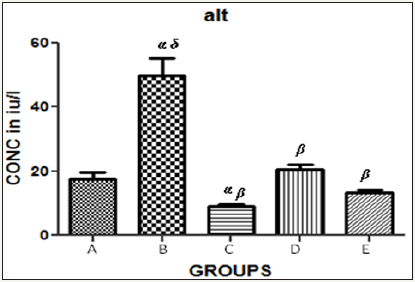
Figure 4 The graph showing the packed cell volume of the rats across the group after treatment.
Results presented as mean±SEM(n=5); α: Significantly different from normal control at p<0.05; β: Significantly different from toxic control at p<0.05; δ: Significantly different from C,D and E at p<0.05
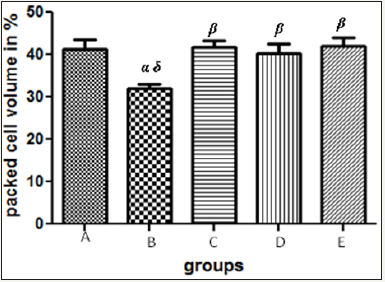
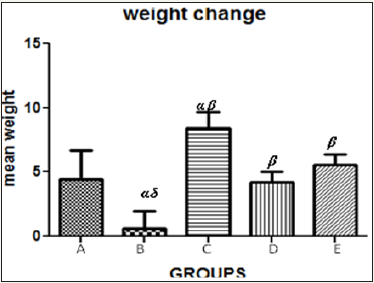
Figure 5 The graph showing the mean weight change of the rats across the group after treatment.
Results presented as mean±SEM(n=5); α: Significantly different from normal control at p<0.05; β: Significantly different from toxic control at p<0.05; δ: Significantly different from C,D and E at p<0.05
Discussion
Serum hepatobiliary enzymes, are present in high concentrations in the liver in stressful conditions. When there is hepatocyte necrosis or membrane damage, these enzymes are released into the circulation, as indicated by elevated serum enzyme levels. Zn was not able to decrease the levels of AST, ALT and ALP as compared to the standard drug essentiale forte but when combined, the enzyme levels were markedly reduced. Significant reduction in the levels of these enzymes in the double treated groups indicated that Zn in combination with Essentiale forte was able to offer better protection to the liver against CCl4 induced hepatotoxicity.
Zn treatment was able to ameliorate CCl4- induced hepatocellular damage as evidenced by reversal of increased serum transaminase (AST and ALT) levels subsequent to exposure. The enzyme levels in group B was found to be markedly elevated to almost three folds however, this enzyme level was quickly reverted to normal after treatments. Moreover, the finds of this research was contrary to the result of the work by Alumot et al. [9] which reported no significant effects on serum enzyme levels or hepatic fat content of rats exposed to doses of CCl4. CCl4 toxicity was also found to have led to relative reduction in the haematocrit in group B unlike the other groups which is in keeping with the work done by Guild et al.[10] & Stewart et al. [11] which found that focal hemorrhagic lesions in the gastrointestinal tract and mild anemia were observed in humans who have ingested Carbon tetrachloride but this is likely due to decreased hepatic synthesis and or secretion of clotting factors. However it is contrary to the finds of Hayes et al. [12]. Oral exposure of mice to carbon tetrachloride did not result in any consistently significant hematological change. Moreover, the work done by Bruckner et al. [13] stated that; severity of hepatic lesions as evidenced by centrilobular fatty vacuolization sometimes with single cell necrosis was correlated with the level of increase in serum enzyme levels [14-64].
Conclusion
Combined administration of Zn and Essentiale forte may be considered as more potentially and synergistically therapeutic through deterrence of enzyme leakage mechanism and thereby inhibiting liver toxicity induced by CCl4. Zn and or Essentiale forte® has hepatoprotective activity against carbon tetrachloride-induced liver damage, this activity could be due to the presence of flavinoids in EPL and membrane stabilization ability in both, thereby preventing cellular leakage. However, further studies are needed to expound the possible prophylactic and or protective effect of the zinc sulphate and essential forte combination in managing chronic liver disease clinically.
Recommendation
Further studies are recommended to give details about the histomorphometric correlation of hepatocytes and kupffer cells in liver of rats treated with CCl4 vis a vis hepatic damage, possible relationship between the zinc level and the degree of liver damage via Zinc bioassay and possible prophylactic effect of the Zinc Sulphate and Essential forte® combination in managing chemicalinduced injury in liver disease.
References
- Cotran RS, Vinay K, Tucker C, Robbins SL (1999) Robbins pathologicbasis of disease. Philadelphia, USA.
- Walker BL, Cooper CD (1992) Air pollution emission factors for medical waste incenerators. J Air Waste Manag Assoc 42(6): 784-791.
- Bojuwoye BU (1997) The burden of viral hepatitis in Africa. West Afr J Med 16(4): 198-203.
- World Health Organization (1998) International programme on chemical safety (IPCS). Health and safety guide no. 107. Geneva.
- Berger A (2002) What Does Zinc Do? BMJ 325(7372): 1062.
- Yadrick MK, Kenney MA, Winterfeldt EA (1989) Iron, copper and zinc status: Response to supplementation with zinc or zinc and iron in adult females. Am J Clin Nutr 49(1): 145-150.
- Zhou Z, Sun X, Lambert JT, Kang JY (2010) Metallonein-independent zinc protection from alcoholic liver injury. Am J Pathol 160: 2267-2274.
- Pilette C, Rousselet MC, Bedossa P, Chappard D, Oberti F, et al. (1998) Histopathological evaluation of liver fibrosis: quantitative image analysis vs semi-quantitative scores. Comparison with serum markers. J Hepatol 28(3): 439-446.
- Alumot E, Nachtomi E, Mandel E, Holstein P (1976) Tolerance and acceptable daily intake of chlorinated fumigants in the rat diet. Food Cosmet Toxicol 14(2): 105-110.
- Guild WR, Young JV, Merrill JP (1958) Anuria due to carbon tetrachloride intoxication. Ann Intern Med 48(6): 1221-1227.
- Stewart PA, Lee JS, Marano DE, Spirtas R, Forbes CD, et al. (1991) Retrospective cohort mortality study of workers at an aircraft maintenance facility. II. Exposures and their assessment. Br J Ind Med 48(8): 531-537.
- Hayes JR, Condie LW, Borzelleca JF (1986) Acute, 14-day repeated dosing, and 90-day subchronictoxicity studies of carbon tetrachloride in CD-l mice. Fundam Appl Toxicol 7(3): 454-463.
- Bruckner JV, Ramanathan R, Lee KM, Muralidhara S (2002) Mechanisms of circadian rhythmicity of carbontetrachloride hepatotoxicity. J Pharmacol Exp Ther 300(1): 273-281.
- Abraham P, Wilfred G, Catherine SP (1999) Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clin Chim Acta 289(1-2): 177-179.
- Adams EM, Spencer HC, Rowe VK, McCollister DD, Irish DD (1952) Vapor toxicity of carbon tetrachloride determined by experiments on laboratory animals. AMA Arch Ind Hyg Occup Med 6(1): 50-66.
- Adaramoye OA, Akinloye O (2000) Possible protective effect of kolaviron on CCl4-induced erythrocyte damage in rats. Biosci Rep 20(4): 259-264.
- Arezzini B, Lunghi B, Lungarella G, Gardi C (2003) Iron overload enhances the development of experimental liver cirrhosis in mice. Int J Biochem Cell Biol 35(4): 486-495.
- Ashe WF, Sailer S (1942) Fatal uremia following single exposure to carbon tetrachloride fumes. Ohio State Med J 38: 553-555.
- Bancroft J, Stevens A (1982) Theory and Practice of Histological Techniques. Churchill-Livingston, USA.
- Barnes DG, Dourson M (1988) Reference dose (RfD): Description and use in health risk assessments. Regul Toxicol Pharmacol 8(4): 471-486.
- Belyaev ND, Budker VC, Deriy LV, Smolenskaya IA, Subbotin VM (1992) Liver plasma membrane-associated fibroblast growth: Stimulatory and inhibitory activities during experimental cirrhosis. Hepatology 15(3): 525-531.
- Bogen KT (1990) Risk extrapolation for chlorinated methanes as promotersvs initiators of multistage carcinogenesis. Fundam Appl Toxicol 15(3): 536-557.
- Bartosiewicz MJ, Jenkins D, Penn S, Emery J, Buckpitt A (2001) Unique gene expression patterns in liver and kidney associated with exposure to chemical toxicants. J Pharmacol Exp Ther 297(3): 895-905.
- Brondeau MT, Coulais C, de Ceaurriz J (1991) Difference in liver and serum malathion carboxylesterase and glucose-6-phosphatase in detecting carbon tetrachloride-induced liver damage in rats. J Appl Toxicol 11(6): 433-435.
- Carson F, Histotechnology (1990) A self-instructional text, ASCP Press, USA, pp. 142-143.
- Crookham,J, Dapson R (1991) Hazardous Chemicals in the Histopathology Laboratory.
- David A, Frantik E, Holusa R (1981) Role of time and concentration on carbon tetrachloridetoxicity in rats. Int Arch Occup Environ Health 48(1): 49-60.
- Driscoll TR, Hamdan HH, Wang G (1992) Concentrations of individual serum or plasma bile acids in workers exposed to chlorinated aliphatic hydrocarbons. Br J Ind Med 49(10): 700-705.
- Elkins HB (1942) Maximal allowable concentrations. II. Carbon tetrachloride. J IndHyg Toxicol 24: 233-235.
- Forbes JR (1944) Carbon tetrachloride nephrosis. Lancet 247(6323): 590-592.
- Fowler JSL (1969) Carbon tetrachloride metabolism in the rabbit. Br J Pharmacol 37(3): 733-737.
- Heineman EF, Cocco P, Gomez MR, Dosemeci M, Stewart PA, et al. (1994) Occupational exposure to chlorinated aliphatic hydrocarbons and risk of astrocytic brain cancer. Am J Ind Med 26(2): 155-169.
- Japan Bioassay Research Center. 1998. Subchronic inhalation toxicity and carcinogenicity studies of carbon tetrachloride in F344 rats and BDF1 mice (Studies Nos. 0020, 0021, 0043, and 0044). Kanagawa, Japan Industrial Safety and Health Association, Japan Bioassay Research Center (Unpublished report to the Ministry of Labor). Hirasawa Hadano Kanagawa, 257 Japan.
- Kazantzis G, Bomford RR (1960) Dyspepsia due to inhalation of carbon tetrachloride vapor. Lancet 13(1): 360-362.
- Leeder JS, Kearns GL (1997) Pharmacogenetics in pediatrics: Implications for practice. Pediatr Clin North Am 44(1): 55-77.
- Luna L (1968) Manual of Histologic Staining Methods of the AFIP. McGraw-Hill, USA, pp. 94-95.
- Jaeger RJ, Conolly RB, Murphy SD (1975) Short-term inhalation toxicity of halogenated hydrocarbons. Arch Environ Health 30(1): 26-31.
- Kogure K, Ishizaki M, Nemoto M, Kuwano H, Makuuchi M (1999) A comparative study of the anatomy of rat and human livers. J Hepatobiliary Pancreat Surg 6(2): 171-175.
- Magos L, Snowden R, White IN, Butler WH, Tuffery AA (1982) Isotoxic oral and inhalation exposure of carbon tetrachloride in Porton-Wistar and Fischer rats. J Appl Toxicol 2(5): 238-240.
- Marchand C, McLean S, Plaa GL (1970) The effect of SKF 525A on the distribution of carbon tetrachloride in rats. J Pharmacol Exp Therap 714(2): 232-238.
- McGuire LW (1932) Carbon tetrachloride poisoning. J Am Med Assoc 99: 988-989.
- McDermott WV, Hardy HL (1963) Cirrhosis of the liver following chronic exposure to carbon tetrachloride. J Occup Med 5: 249-251.
- Nagano K, Nishizawa T, Yamamoto S (1998) Inhalation carcinogenesis studies of six halogenated hydrocarbons in rats and mice. In: Chiyotani K, Hosoda Y, Aizawa Y (Eds.), Advances in the prevention of occupational respiratory diseases, Elsevier Science, pp. 741-746.
- New PS, Lubash GD, Scherr L, Rubin AL (1962) Acute renal failure associated with carbon tetrachloride intoxication. JAMA 181: 903-906.
- Norwood WD, Fuqua PA, Scudder BC (1950) Carbon tetrachloride poisoning. Arch Ind Hyg Occup Med 1: 90-100.
- Paustenbach DJ, Clewell HJ, Gargas ML, Andersen ME (1988) A physiologically based pharmacokinetic model for inhaled carbon tetrachloride. Toxicol Appl Pharmacol 96(2): 191-211.
- Prendergast JA, Jones RA, Jenkins LJ (1967) Effects on experimental animals of long-terminhalation of trichloroethylene, carbon tetrachloride, l,l,l-trichloroethane, dichlorofluoromethane, and l,ldichloroethylene. Toxicol Appl Pharmacol 10: 270-289.
- Riley TR, Bhatti AM (2001) Preventive strategies in chronic liver disease: part II. Cirrhosis. Am Fam Physician 64(10): 1735-1740.
- Sakata T, Watanabe A, Hobara N, et al. 1987 Chronic liver injury in rats by carbon tetrachloride inhalation. Bull Environ ContamToxicol 38(6): 959-961.
- Sanzgiri UY, Bruckner JV (1997) Effect of emulphor, an emulsifier, on the pharmacokinetics and hepatotoxicity of oral carbon tetrachloride in the rat. Fundam Appl Toxicol 36(1): 54-61.
- Sheehan D, Hrapchak B (1980) Theory and practice of Histotechnology. Battelle Press, USA, pp. 189-190.
- Shen ES, Garry VF, Anders MW (1982) Effect of hypoxia on carbon tetrachloride hepatotoxicity. Biochem Pharmacol 31(23): 3787-3793.
- Siegers CP, Horn W, Younes M (1985) Effect of hypoxia on the metabolism and hepatotoxicity of carbon tetrachloride and vinylidene chloride in rats. Acta Pharmacol Toxicol 56(2): 81-86.
- Smyth HF, Smyth HF, Carpenter CP (1936) The chronic toxicity of carbon tetrachloride; animal exposure and field studies. J Ind Hyg Toxicol 18: 277-298.
- Teschke R, Vierke W, Gellert J (1984) Effect of ethanol on carbon tetrachloride levels and hepatotoxicity after acute carbon tetrachloride poisoning. Arch Toxicol 56(2): 78-82.
- Tomenson JA, Baron CE, O’Sullivan J, Edwards JC, Stonard MD, et al. (1995) Hepatic function in workers occupationally exposed to carbon tetrachloride. Occup Environ Med 52(8): 508-514.
- Watanabe T, Niioka M, Hozawa S, Kameyama K, Hayashi T, et al. (2000) Gene expression of interstitial collagenase in both progressive and recovery phase of rat liver fibrosis induced by carbon tetrachloride. J Hepatol 33(2): 224-235.
- Weber L, Boll M, Stampfl A (2003) Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33(2): 105-136.
- Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA (2002) Effect of prolongedvigabatrin treatment on haematological and biochemical parameters in plasma, liver, and kidney of Swiss albino mice. Sci Pharm 70: 127-137.
- Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, et al. (2002) A 90-day oral gavage toxicity study of D-methylphenidate and D,Lmethylphenidate in Sprague-Dawley rats. Toxicology 179(3): 183-196.
- Tofovic SP, Jackson EK (1999) Effect of long-term caffeine consumption on renal function in spontaneously hypertensive heart failure prone rats. J CardiovascPharmacol 33(3): 360-366.
- Klaassen CD (2001) Casarett and Doull’s toxicology: the basic science of poisons. McGraw-Hill, NewYork, USA.
- Martelli A, Mereto E, Ghia M (1989) Protective effect of phosphatidylcholine on hepatic lipid peroxidation in rats. Med Sci Res 17: 995-996.
- Guiliano L, Loche A, Ottonello M (1987) Evaluation of the protective activity of phosphatidylcholine in comparing lipid peroxidation processes. Boll Soc Ital Biol Sper 63: 481-487.
© 2018 Arayombo Babatunde E. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






