- Submissions

Full Text
Novel Techniques in Nutrition and Food Science
Potential Utilization of Fruit and Vegetable Wastes for Food through Drying or Extraction Techniques
Nora Salina Md Salim1*, Ashutosh Singh2 and Vijaya Raghavan3
Council for Agricultural Research and Economics, Research Centre for Olive, Italy
1School of Fundamental Science, Universiti Malaysia Terengganu, Malaysia
2School of Engineering, University of Guelph, Canada
3Department of Bioresource Engineering, McGill University, Canada
*Corresponding author: Nora Salina Md Salim, School of Fundamental Science, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia
Submission: October 30, 2017; Published: December 12, 2017

ISSN 2640-9208 Volume1 Issue2
Abstract
Reducing food waste by complete utilization of resources creates good impact for the food security, economy, and the climate. Current world population is projected to rise to 11.2 billion by the end of this century. With the incremental population, more food needs to be produced to fulfill the future demand. However, one-third of food produced is lost or wasted globally, and these wastes contribute to global climate change. Fruit and vegetables, are highly perishable biological materials contributed to the higher food waste compared to other commodity. Therefore, this paper identifies the potential utilization of abundance of seasonal crop, crop remains, and also by-products of fruit and vegetable using appropriate technologies for the conversion of food wastage into value added products. Both techniques can help to facilitate the effective food production to feed the world.
Keywords: Food losses; Food waste; Food security; Crop remains; By-product; Drying; Extraction
Introduction
About 1.3 billion tons per year of food is lost or wasted, starting at the production stage and ending at the consumer domain [1]. At the same time, United Nations predicts that food demand must be increased by 70 % to feed the projected world population by 2050. Presently, the world population of 7.3 billion is expected to be increased to more than 9.7 billion by 2050 and according to the 2015 Revision (United Nations), the population seems to continue to grow by almost 30 million per annum now.
Figure 1: World population at different major areas in the year of 2015, 2030 and 2100. United Nations (2015).
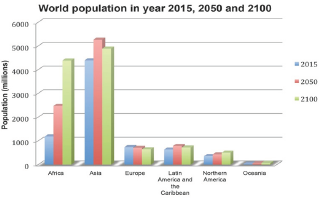
Figure 1 illustrates the current and the projected world population at major areas. From this figure, it is seen that nearly all the population growth is take place in the developing countries. According to these provided revised population data, the world continues to face a challenge to have an adequate supply of food to feed the fast growing population. This means, we need to produce more food with the existing limited natural resources (land, water, energy and fertilizer).
The potential of reducing waste to combat the food security issues are high, as Lipinski [2] estimates that by 50 % reduction in current food waste, the world would be saving 1314 trillion kcal per year. This represent a reduction of about 22 % of the number of additional calories needed to feed the projected population by 2050. Moreover, reduction in food waste lead to more efficiency in economic productivity and reductions in emissions of greenhouse gases that contributes to climate change [WRAP, 2015]. Furthermore, Foley [3] highlighted the reduction of waste and utilization of resources more efficiently. If one can utilize food produced effectively, it would help in world food security in addition to contributing towards the reduction of environmental impact of agriculture.
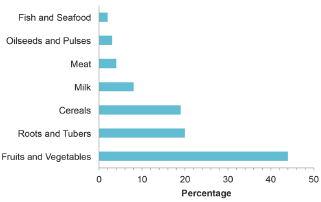
The amount of fruit and vegetable are not only increased due to the demand in consumption but it was also found to be the largest food waste contributor. Figure 2 presents the estimated global food waste from different food commodities. Fruits and vegetables waste of represent 44%, roots and tubers contributing by 20%, cereal by 19% and other commodities also produce significant amount of waste. For example, fruits and vegetables are commonly produced seasonally and overproduction during the season without proper utilization leads to the food waste, especially in tropical countries. In addition to that, crop remains commonly the plant parts like stem, stalks and leaves are plowed back to the field although they contains sufficient nutritional ingredients. As in food processing industry, when the raw materials enter the production process it produces the desired products and also the waste. It is estimated that 25-30% waste produced from the processing of fruits and vegetables in the form of pomace, peels, and seeds [4].
Figure 2: Global food waste by commodity. FAO [1].
Due to these compelling circumstances, researchers are working towards reducing the food waste and identifying the potential crop remains and by-products that contain nutritional ingredients which are needed for the population; this leads to the domain of new value added products at the market place [5- 8]. In other words, transformation of these wastes to food can have positive effects towards the sustainable future. The aim of this study is to provide brief overview on the potential food resources from fruit and vegetable wastes and the innovative transformation opportunities using appropriate technology like drying and extraction technique for better utilization of valuable organic material.
The Potential Food Resources at Selected Fruit and Vegetable Wastes
Crop remains in fruits and vegetables production is commonly from the stem, stalks, and leaves of the plants. Such examples are common in broccoli industry. Broccoli is a nutritious vegetable belonging to the Cruciferae family and the genus Brassica and has been consumed worldwide. It has been conclusively shown that consumption of broccoli can help reduce the risk for the development of certain forms of cancer [9,10]. Commonly, broccoli products are predominantly from the florets part, which only represent about 25% of to the total mass plant. The leaves and stalks are left as crop remains. Campas-Baypoli [11] found that the stalks have higher total carbohydrate and crude fiber compared to leaves and florets. Table 1 shows the comparative nutritive value between floret and stalk of broccoli and it can be seen that the stalk still contains nutritional value in them that can be further explored for product development rather than farmers plowing back the crop remain into the soil.
Table 1: Nutritional value of broccoli floret and stalk [33].
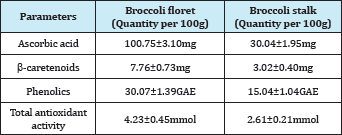
*GAE=Gallic acid equivalent
Most of the fruit plants are cultivated for its fruits. However, the leaves of the fruit plants also contain nutritional compounds to be explored for food products. Blueberry (Vaccinium sps) is one of the popular fruit in North America and was known for their health beneficial effects such as anti-diabetic, anti-bacterial and anti-cancerous properties [12-14]. The most commercial blueberry species are high bush blueberry (Vaccimium corymbosum) and low bush blueberry (Vaccinium ausuttifolium) (Retamales & Hancock, 2012). In Canada, blueberries are the largest fruit export commodity with 62% of total value and 44.8% of total volume [15]. The leaves of blueberry, which are usually been wasted during mechanical harvesting, have been found to be rich sources of phenolic compounds, anthocyanins and chlorogenic acid which are known for its antioxidant properties [16-18]. Therefore, utilization of those leaves has a good potential to produce commercial food products.
Fruit seasons mirrored the abundance of tropical fruits especially in Southeast Asia and the falling prices for crops. Therefore, more commercial value added products could be produced to combat the issue of overall return to the producer. Durian (Durio zibethinus L.) is widely known as the "king of tropical fruits", belonging to the family Malvaceae. Durian is a good source of carbohydrates, proteins, fats, vitamin and potassium. However, the fruit have a short shelf life which is between 2 to 5 days when stored at room temperature [19]. Another example of an abundant tropical fruits is rambutan (Nephelium lappaceum L.). Rambutan is oval in shape and hairy on the outside and grows in warm tropical climates. Rambutan contains high vitamin C concentration at about 36.4mg/100g [20]. However, it has a short shelf life of 3 to 4 days at room temperature [21]. There is a great potential to produce value added products with longer shelf stability.
Furthermore, guava (Psidium guajava L.) known as the "apple of tropics", grown in tropical and subtropical countries [22], is often marketed as a "super fruit", being rich in vitamins A and C and also has good level of the dietary minerals, potassium, and magnesium [23]. Meanwhile, the leaves of guava plant are traditionally used in folk medicine in Mexico, Latin America and the Caribbean. Laily [24] reported that guava leaves contains high level of antioxidant, phenolic compounds and immune-stimulatory agents for commercialization. Its potential in value-added industry is known to be quite high.
Waste and by-products from fruits and vegetables processing that still contains potential bioactive components have been considered for waste minimization and recovering the value through transformation to products. Apple (Malus domestica Borkh.) is a deciduous fruit that consumed worldwide as fresh and processed. The world production of apple has increased by more than 10 million tonnes between 2009 and 2013 with China as a major producer, followed by the United States of America, Turkey, Poland and Italy [25]. In apple juice processing, the pomace or apple press cake is the major waste discarded, which contains flesh, skin, seeds and stem [26,27]. Study done by Bhushan [28] shows that apple pomace produced from apple juice processing contains various polyphenols including flavanols, chlorogenic acids and glycosides. However, the low content of vitamins and proteins in apple pomace with high content of pectin can lead to its use as raw material for production of pectin [29]. Furthermore, abundant of apple peel waste are produced from applesauce, canned and dried apple industry [30,31]. Study conducted by Łata and Tomala [32] found that apple peel contains higher amount of flavonols, ascorbate and total phenolics and thus, it requires an innovative technology to provide more value added products from this waste. For example, apple peel powder may be used in various food products to add phytochemicals and promote good health [31]. Hence, there is an urgent need to develop a new processing line to deal with this resource.
Potato (Solanum tuberosum L.) is one of the largest processed vegetables generating over 4.3 million tonnes of co-products in the USA and Canada as in 2008 [33]. In the same year, over 21.8 million tonnes of potatoes were produced of which over 50% were processed mainly for French fries (60%) and chips (22%). Wastes generated from potato processing are estimated to be about 33 to 35% of the fresh weight [33]. Large proportion of this waste is used as animal feeds and for the production of first-generation biofuels. In recent years several studies have shown that potato peels, which consist of a major proportion of potato processing industry waste, can be used as a natural source of antioxidants [33]. The recovery of these valuable components from potato peel waste can provide a cheaper alternative to synthetic antioxidants that are commercially utilized in the food industries.
Another example is grape. Grape (Vitis vinifera L.) is one of the largest fruits crop in the world with production of over 69 million tonnes per year [25]. Grape pomace is a by-product of wine production and consists of skin, seeds, pulp and stalks [34]. Pomace is a rich source of bioactive compounds, such as polyunsaturated fatty acids (PUFA), polyphenols, dietary fiber, and flavonoids including catechins and proanthocyanidins [3537]. Grape seeds constitute a considerable proportion of the pomace amounting to approximately 30-40% on a dry basis [38]. Oil extracted from grape seeds is rich in polyunsaturated fatty acids (PUFA), especially linoleic acid. It is also appreciated for the content of various procyanidins and phenolic compounds including gallic acid, catechin and epicatechin [39]. Extensive research has demonstrated that extracts from grape seeds and skin have several potentially beneficial effects on human health, such as antioxidant and radio protective effects [40], antihyperglycemic effects [41], prevention of hypertriglyceridemia by improving insulin sensitivity and anti- inflammatory effects [41].
5. Conventional and Emerging Technologies for Conversion of Food Wastage into Value Added Products
As pointed out in previous section, it is clear by now that crop and harvest remains, the abundance of seasonal produce, by-products and waste from the food production and processing still contain nutritional ingredients in them that can be used for food. Therefore, the product innovations using appropriate technologies are required in the transformation of this edible waste for consumption and thus, help in reducing overall food wastage problems. There are several potential food products, which can be considered for the waste transformation such as food additives, colorant and flavoring agents. However, in this review, only dehydrated products and nutraceuticals products were given primary consideration.
Dehydrated products using drying methods
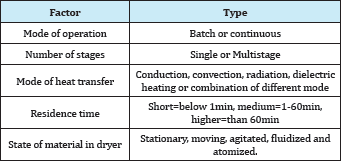
Edible waste from crop remains and abundance of seasonal fruits can be promoted as dried products. Drying is one of the key unit operation required for producing a newer, acceptable and excellent edible food products. This water removal process is aimed to reduce the water activity of the perishable biological materials in order to produce a shelf stable product. Commercially, dried products from fruit and vegetables are used as confectionary products, flour, flakes, granulated, powder, additional ingredient of ready-to-eat soup, salads, energy bars, and cereals, and also as snacks products. The diversity of food products has spurred the various type of dryer around the world. The classifications of the dryer system are as listed in Table 2 and the most prevalent ones are based on the mode of heat transfer.
Table 2: Classification of dryer system [52].
Traditionally in developing countries, that receive the greatest amount of sunlight throughout the year, open sun drying is a method practised to obtain lower moisture content of food products. Direct sun radiation is a simple and low-cost operation method but requires open large space, susceptible to foreign contamination of materials such as dirt, rodent, insects and birds and is highly dependent on the availability of sunshine [42]. The problem with the open sun drying is that, when sudden change in the weather such as cloudy and rainy days, it leads to improper drying and eventually affects the quantity and quality of the crop produce. Direct exposure with uncontrolled temperature from the open sun during drying may also lead to non-uniform drying and quality degradation (e.g. case hardening). However, this drying method can be improved by providing a raised platform covered with netting as protection from the dirt, animal and insects. The quality of sun- dried foods can be enhanced by reducing the geometry size of the food product to achieve faster drying [43].
The alternative of the open sun drying is to use the solar dryer system. In solar dryer, the sun radiation is absorbed by the solar collector, converts it into heat and thenceforth the heat is transfer to a flowing fluid (usually air, water, or oil) and passed through food in the drying chamber. Solar collector is the major component in energy transfer conversion and energy transfer in the solar dryer system. Thus, a considerable amount of literature has been published on the innovation of solar collectors such as by applying selective coatings, artificial roughness and the application of nanofluids as working fluid for enhancing the thermal performance of solar collectors [44,45]. Apart from that, there are also on-going research on hybrid solar drying system in order to have the ability to operate without solely depending on the sun, reduces chance of food loss and the capacity to operate at higher drying temperatures [46]. Solar food dryers are efficient low-cost systems and they can provide faster drying and hygienic and healthier dried products when compared to open sun drying [47,48].
Application of hot air as a carrier of heat is mostly used in drying process due to its simplicity. The concept uses air heated and being forced through the layer of product in a closed system. For example, tray dryer are the common dryer designed for uniform circulation of hot air in the dryer chamber where the food is spread out on perforated trays. These dryers are the least-expensive dryer type however it required longer drying time (usually 12 to 48h) to dry the materials [49]. Further, vacuum dryers are similar to tray dryer, expect the system is operated under vacuum conditions. In vacuum dryer, heat is supplied by conduction (hot oil, heated elements, etc.), sometimes by radiation or by steam. These drying offer product- specific advantages such as low-temperature drying, because evaporation of water proceeds at temperatures as low as 30 °C and low oxidation to the products [50,51]. However, the operation of vacuum drying system is expensive and thus it is recommended only for heat-sensitive materials [52].
The most common type of dryer used to produce dry particulate products is the fluidized-bed dryer. Fluidized conditions are achieved when airflow passes upward through a bed of particles at sufficient velocity to overcome the gravitational forces on the particles. A variant of fluidized bed is the spouted bed drying. Spouted bed drying has been widely used for large particles (>5mm) where the airflow rates required for fluidization are very high [53]. The advantages of these dryers are [54]: (1) high drying rate due to excellent gas-particle contact leading to high heat and mass transfer rates; (2) smaller flow area; (3) high thermal efficiency; (4) easy to implement; (5) lower capital and maintenance costs; and (6) ease of control.
Spray dryer can be used to produce dried materials in the form of powder, granules or agglomerates depending on the physical and chemical properties of the solution feed and the operating conditions of the spray dryer [51]. Solution feed entering the spray dryer is atomized and the droplets formed are contacted with a heated air and thus evaporate the moisture and leaving a dry powder product [55]. This drying method is appropriate for production of vegetable and fruit powders. However during the drying process, sugars such as sucrose, glucose, fructose and organic compounds like citric, malic and tartaric acids present in such materials have low molecular weights that results in low glass transition temperature and thus contribute to the stickiness problem [56]. Stickiness problem is still the major impediments to mass production of fruit and vegetable powders. Thereby, several approach have been made to overcome this issue such as by addition of high molecular weight carrier agents (e.g. maltodextrin, gum-Arabic) to increase the glass transition temperature of feed mixture, use low humidity and low temperature drying conditions, and by modifying the surface properties of the atomized droplets thru the addition of proteins such as whey, casein and soy protein [57,58].
In recent years, microware drying has received considerable attention due to the improved drying kinetics, energy saving and quality of the dried product. Microwaves generate heat by exciting dipolar and ionic molecules by the alternating electromagnetic field resulting in rapid heating, which is known as volumetric heating, from within the sample (Orsat et al., 2007; Wray & Ramaswamy, 2013). However, from an economic point of view, stand-alone microwave drying is very costly. Hence, combination of microwaves with other drying technique such as hot air or vacuum is recommended [47]. For instance, microwave-assisted hot air drying was found to be an applicable approach that can minimize the retreating wet front, maintaining the evaporating surface and perhaps permitting entrainment- evaporation during the drying process [59]. Comparative study conducted by Alibas (2007) shows that combined microwave with hot air drying of pumpkin slices offer a convenient drying method in terms of energy consumption, drying period and color criteria compared to stand alone microwave drying and hot air drying. Dev [60] studied the drying kinetics of drumstick tree (Moringa oleifera) under microwave assisted hot air drying. They found that the drying time were reduced by more than 80% and the product quality was better in terms of color, rehydration rate and low retention of volatiles when compared to hot air drying. In general, microwave assisted drying can meet the four major requirements in drying foods which is speed of operation, energy efficiency, cost of operation and quality of dried products.
From all the above-mentioned drying methods, the water removal is accomplished based on the phase change from liquid to vapor, which different from freeze-drying. Freeze-drying is a method in which water is removed from the material by sublimation. Sublimation is the process where the frozen water changes directly into vapor under vacuum conditions. The freeze- dried products are very light and crispy; retain much of their original flavor (taste and aroma) and phytochemicals. However, the process is long and expensive than the atmospheric drying but the quality of the product is considered as superior to any other dehydration techniques.
There is also consensus that by applying osmotic dehydration prior to drying process can reduce the energy requirement which is a major concern in drying process in terms of economic consideration [59]. Osmotic dehydration is regarded as the partial removal of water in liquid form (without a phase change) from biological materials by immersion in osmotic solution. Besides lowering the energy consumption, osmotic pre-treatment prior to drying can shorten the drying time and improves overall product quality such as color, texture, aromas and also nutritional values [61-63].
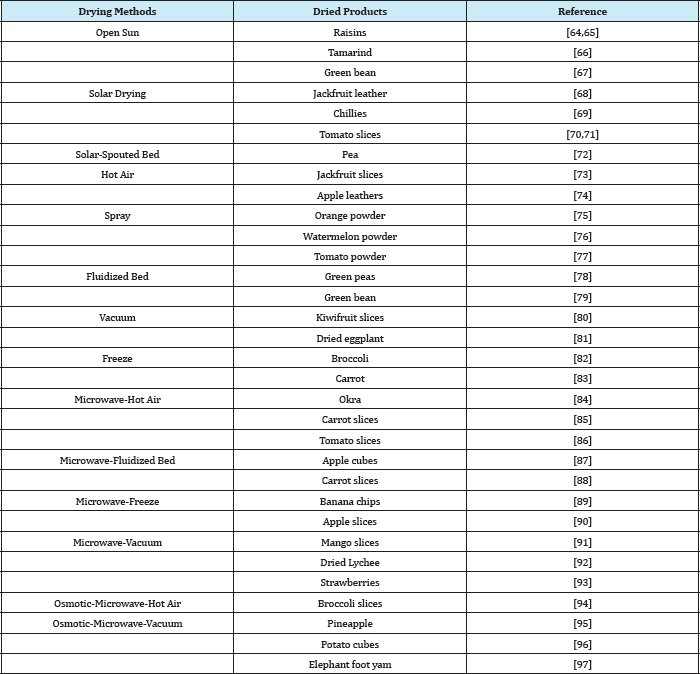
Nevertheless, there is no one technique of drying that is applicable for all products because each biological material has their own unique properties and hence the requirements are varied. Thus, there are many studies conducted for various fruits and vegetables using different drying techniques to produce desired dried products as listed in (Table 3). The developments of new attractive dehydrated products using the innovative drying technologies will increase and diversify its availability, minimize the food losses and help better economic return for the producers.
Table 3: Different drying technique used for production of variety of dried products.
Nutraceuticals products using extraction methods
Several ancient philosophers have referred food as a natural medicine. For several years the scientific community has been involved in identification of essential elements in food such as vitamins, minerals and proteins to prevent various dietary deficiency diseases, commonly termed as "under nutrition" [98]. With several decades of research, clinical trials and technological advancements scientists and food processors have been able to start a trend of food products now known as "functional foods" and "nutraceuticals". The terms functional foods and nutraceuticals do not have a universally acceptable definition. However, for this review the definition of functional foods and nutraceutical stated by Health Canada has been considered [99]. They define nutraceutical as "a product isolated or purified from foods that are generally sold in medicinal forms not usually associated with food". A nutraceutical is demonstrated to have physiological benefits or provide protection against chronic disease. Functional food is termed as "a food similar in appearance to, or may be, a conventional food, which is consumed as part of a usual diet, and is demonstrated to have physiological benefits and/or reduce the risk of chronic diseases beyond basic nutritional functions".
Wastes from fruits and vegetables are rich in phenolic compounds, which are low molecular weight plant secondary metabolites and are chemically very heterogeneous. They generally comprise of polyphenols, carotenoids, alkaloids, saponins, etc. Polyphenols are classified into flavonoids, xanthones and phenolic acids including hydroxybenzoic and hydroxycinnamic acids [100]. These food wastes contain potentially marketable components and it is in favor of food industries to exploit the high value components such as proteins, polysaccharides, fibers, flavor compounds and phytonutrients as functional ingredients for food and pharmaceutical products [101]. These components need to be separated/extracted from the food wastes through combined chemical, physical and biochemical approaches. This has been the object for several research activities [101-104]. The primary motive of extraction of valuable components from wastes is to be economically viable, i.e. the processes selected for extraction of components have to be selective and highly effective in extracting marketable components [105]. Selection of appropriate extraction technique is important due to their effect on the quality and yield of compounds from waste matrices into the extracting medium [106].
Conventionally the bioactive compounds are extracted from their sources using traditional solid- solvent extraction technique such as Soxhlet, which requires removal of high value compounds using water and organic solvents including methanol, ethanol, hexane, acetone, etc. Traditional techniques are time consuming, have lower yield and require large volumes of organic solvents that make them both uneconomical and unsustainable. With advancements in technologies, late 1900s saw development of novel extraction techniques such as microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), pulsed-electric field (PEF) assisted extraction, cavitation (hydrodynamic), etc. Introduction of these techniques shortened extraction time, increased yield and reduced the overall consumption of organic solvents.
The different stages in the extraction of nutraceutical compounds are: pre-treatment, extraction, isolation/purification and encapsulation. The pretreatment step includes homogenization, grinding, milling, maceration and drying. The extraction step is the most crucial and has the maximum impact on the overall economics of the value-added product development process. Selection of the appropriate extraction method depends on several factors such as the type of bioactive compounds that are being extracted, desired yield, operational requirements and overall cost. Extraction of value-added components from food wastes requires careful clarification of wastes, i.e. food wastes can be clarified on the basis of their source, in low-income countries food wastes are primarily comprised of agricultural produce generated during postharvest handling processes.
Table 4: List of a few bioactive components extracted from various food waste sources.
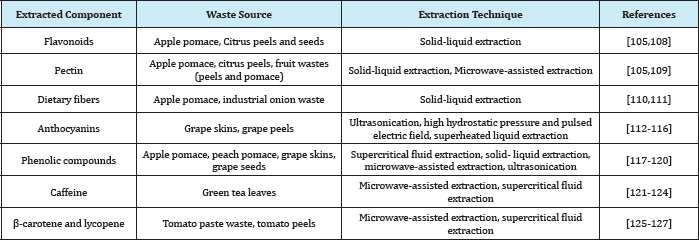
Fruits and vegetables are generally the most widely investigated substrates for the extraction of phenolic compounds and other bioactive components such as proteins, dietary fibers (Table 4). Processing of these wastes progresses from a macro to micro scale, i.e. the macro scale includes the pre-treatment, which as stated earlier is required to prepare the material for downstream extraction process. Most fruits and vegetables wastes require clarification, for example in case of grape pomace, clarification of pomace allows separation of individual components such as seeds, pulp and skins [128]. This clarification allows proper selection of pre-treatment processes, such as for grape seeds milling facilitates and improves the yield the extraction of grape seed oil by increasing the contact surface area of the substrate and the extracting solvent. In some cases such as apple pomace, removal of water is required to concentrate the substrate but thermal treatment leads to activation or de-activation of key enzymes, which affect the yield and quality of the target value-added compounds. Application of non-thermal processes such as mechanical pressing and freeze drying can be applied to reduce thermally-induced loss of yield and functionality of bioactive compounds [129,130]. Other processes such as centrifugation and microfiltration have also been suggested as an alternative to thermal processes as they allow selective removal of substrates.
The second stage (extraction) involves use of organic solvents for recovery of value added compounds from clarified substrates (samples). Solvent extraction is a convenient process as it provides a physical carrier for movement of target molecules between different phases. Polar solvents such as ethanol, methanol, water or their mixtures have been widely used for extraction polyphenols from plant sources. Polyphenols readily dissolve in polar solvents, whereas lipids/oils can be extracted using non-polar solvents such as hexane and acetone. Among all solvents ethanol is the most widely preferred because it is cheap and has a "GRAS" (Generally- Recognized-As-Safe according to American Food and Drug Administration) status [4,131]. Solvent extraction can be achieved using traditional methods such as Soxhlet, which requires the reflux of solvent through sample solid-bed multiple times until complete extraction has been achieved. The efficiency of the Soxhlet process depends on several operational parameters, such as sample matrix characteristics, solvent composition and extraction temperature [132, 133].
Another way by which solvent extraction can be accomplished is by using reflux extraction method, in which the sample is mixed with the solvent in a packed bed reactor or an agitated vessel, followed by centrifugation and filtration. This conventional method has been applied for the extraction of potential antioxidants from olive oil mill wastes, which was treated with ethanol at a solvent to solvent ratio of 5:1v/w, pH 2 for 180 minutes [134]. Similarly phenolic antioxidants have also been extracted from winery wastes at a solvent to sample ratio of 9:1v/w and pH 1.5 with extraction time of 180min [135]. The operational parameters including solvent to sample ratio, extraction time and pH play an important role in determining the overall yield of the process but in some cases further steps are required to enhance the extraction yield. For example, in case of pectin and hemicellulose extraction, precipitation with ethanol had to be followed by treatment with alkali or acid [136]. The overall yield achieved using solvent extraction process is often low and of reduced quality because most of bioactive compounds are heat-sensitive and are lost during the extraction process. This process is also time consuming and uses large volume of solvents, which add to overall processing cost and environmental issues due to their disposal.
The drawbacks of Soxhlet extraction process can be mitigated by using the novel extraction techniques such as microwave- assisted extraction (MAE). MAE utilizes microwave energy to rapidly and volumetrically heat the solvent thus accelerating the transfer of the analytes from the sample matrix to the solvent. When microwave passes through the solvent, its energy is absorbed and converted into heat by virtue of the dielectric properties of the solvent. In recent years this technique has been widely applied to extraction antioxidants and other valuable components such as pectin from fruit and vegetable wastes [11,137-142]. The overall performance of MAE process depends on number of factors, including solvent composition, microwave power level selected, extraction temperature, extraction time and sample characteristics. The choice of solvent composition is related to its dielectric properties (dielectric constant and dielectric loss), which in a way is also dictated by its interaction with the sample [135]. Dielectric constant dictates the solvents capability to absorb microwave energy and dielectric loss dictates its ability to convert this absorbed energy into heat [135]. The dielectric property of the selected solvent can be modified by combining it with other solvents [143]. The choice of solvent is also crucial as it determines the extraction temperature. For extraction of thermolabile bioactive components a combination of different solvent is required to lower the dielectric loss factor, this keeps the extracted analytes at a lower temperature and also allows for selective extraction [135]. Due to its advantages such as lower extraction time, reduced solvent usage, enhanced extraction yield and ability to selectively extract value- added bioactive components over conventional solvent extraction method, MAE can be considered as a potential alternative for extraction of antioxidants from fruits and vegetable wastes [144].
At present, application of MAE process in food industry is limited due to stringent food quality and safety regulations. Use of solvents for extraction requires additional purification steps, which adds to the overall cost of the process. To combat these disadvantages another modern technique of supercritical fluid extraction (SFE) technique has gained tremendous interest. SFE utilizes the thermodynamic properties of solvents (e.g. ethane, butane, water, pentane, CO2) at their supercritical point [145]. SFE process works on the principle of distribution of analyte between two different phases namely the separation and stationary phase [146]. SFE has several advantages including high solvating power that can be easily controlled by varying the pressure and temperature during extraction, diffusivity of supercritical fluid is better than solvents which improves extraction yield, it is also non-toxic and is very environmentally friendly [147]. SFE has been industrially applied for recovery of hydroxytyrosol from olive mill waste [148], extraction of antioxidants from Brazilian cherry seeds [149], flavonoids from Oregano leave [150], polyphenols from grape bagasse [151] and anthocyanins from grape peel [152]. The disadvantage of SFE process includes the complexity of the operational parameters and high operation and maintenance costs. Commercialization of SFE process is limited, as it requires several downstream processing steps to obtain a viable product. This limitation can be overcome by integrating SFE process with upstream preprocessing steps such as fractionation, chemical and enzymatic conversion of wastes in-order to make it appropriate for extraction process and post-processing steps such as purification.
Another technique that has garnered interest of scientific communities for extraction of phytonutrients from plant, animal and microbial sources and can be used to extract valuable components from food wastes is ultrasound-assisted extraction (UAE) technique. On a lab scale UAE has been widely used to extract bioactive compounds such as antioxidants and tocols, essential oils and lipids from plants [153-155]. In this process sound waves of frequencies higher than 20 kHz travel through the sample matrix involving an expansion and compression cycles. The expansion cycle pulls the molecules apart and compression brings them together, when sound waves pass through liquids (solvents) they create bubbles that grow and collapse. This phenomenon is also known as cavitation and it disrupts the cell walls and improves the mass transfer of nutrients from cellular matrix into the extracting solvents [156]. The design and fabrication of UAE based extraction system is cheaper as compared to MAE and SFE processes, but is limited in capacity and has a lower yield compared to them [157].
It is important to note that the yield of valuable compounds from food and vegetable wastes or any other sources account for only a small fraction of its total weight; hence the economic feasibility of the process is largely dependent on the extraction yield, quality and its market value. Nutraceutical market is worth billions of dollars and with sustained market demands expected for several years to come it is important to identify a universal process design that can be used to develop and market products developed from food wastes [158,159].
Conclusion
By the year 2100, the global population is expected to increase to 11.2 billion. To feed this projected population and address the food security and the environmental issues, waste reduction and utilization food resources are the important strategies to be developed. The nutritional characterization of waste from fruits and vegetables suggest that most of these crop remains and byproducts can be utilized, recovered and converted into value added food products. Hence, value addition of these wastes through drying technology and extraction methods to dehydrated products and nutraceutical products, respectively could be an alternative market option for the food and the associated industries. This in turn can create more economic opportunities; enhance the social and environmental value for the producers, processors and consumers.
Acknowledgement
The authors are grateful to NSERC (Natural Sciences and Engineering Research Council of Canada) for the financial support of this study and to Ministry of Higher Education Malaysia and Universiti Malaysia Terengganu for the granted scholarship.
References
- FAO (2011) Global food losses and food waste-extent, causes and prevention. UN FAO, Rome.
- Lipinski B, Hanson C, Lomax J, Kitinoja L, Waite R, et al. (2013) Reducing food loss and waste. In: working paper, installment 2 of creating a sustainable food future, world resources institute. Washington, DC, USA, pp. 1-40.
- Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, et al. (2011) Solutions for a cultivated planet. Nature 478: 337-342.
- Galanakis CM (2013) Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food and Bioproducts Processing 91(4): 575-579.
- Ayala-Zavala JF, Vega-Vega V, Rosas-Dommguez C, Palafox CH, Villa RJA, et al. (2011) Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Research International 44(7): 1866-1874.
- Đilas S, Čanadanović-Brunet J, Ćetković G (2009) By-products of fruits processing as a source of phytochemicals. Chemical industry and chemical engineering quarterly 15(4): 191-202.
- Laufenberg G, Kunz B, Nystroem M (2003) Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations. Bioresour Technol 87(2): 167-198.
- Peschel W, Sánchez-Rabaneda F, Diekmann W, Plescher A, Gartzia, et al. (2006) An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chemistry 97(1): 137-150.
- Finley JW (2003) Reduction of cancer risk by consumption of selenium-enriched plants: enrichment of broccoli with selenium increases the anticarcinogenic properties of broccoli. J Med Food 6(1): 19-26.
- Latté KP, Appel KE, Lampen A (2011) Health benefits and possible risks of broccoli-an overview. Food and Chem Toxicol 49(12): 32873309.
- Campas-Baypoli ON, Sánchez-Machado DI, Bueno-Solano C, Núñez- Gastélum JA, Reyes-Moreno C, et al. (2009) Biochemical composition and physicochemical properties of broccoli flours. Int J food sci nutr 60(supp 4): 163-173.
- Chatterjee A, Yasmin T, Bagchi D, Stohs S (2004) Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin. Mol Cell Biochem 265(1-2): 19-26.
- Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, et al. (2009) Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 16(5): 406-415.
- Li C, Feng J, Huang WY, An XT (2013) Composition of polyphenols and antioxidant activity of rabbiteye blueberry (Vaccinium ashei) in Nanjing. J Agric Food Chem 61(3): 523-531.
- Torabi S (2014) Statistical overview of the Canadian fruit industry 2013, market analysis and information section, Horticulture and cross sectoral division, Agriculture and Agri-Food, Canada.
- Hicks JM, Muhammad A, Ferrier J, Saleem A, Cuerrier A, et al. (2012) Quantification of chlorogenic acid and hyperoside directly from crude blueberry (Vaccinium angustifolium) leaf extract by NMR spectroscopy analysis: single-laboratory validation. J AOAC Int 95(5): 1406-1411.
- Kim SM, Shang YF, Um BH (2010) Preparative separation of chlorogenic acid by centrifugal partition chromatography from highbush blueberry leaves (Vaccinium corymbosum L.). Phytochem Anal 21(5): 457-462.
- Routray W, Orsat V (2014) MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Industrial Crops & Products 58: 36-45.
- Ho LH, Bhat R (2015) Exploring the potential nutraceutical values of durian (Durio zibethinus L.) -An exotic tropical fruit. Food Chem 168: 80-89.
- Wall MM (2006) Ascorbic acid and mineral composition of longan (Dimocarpuslongan), lychee (Litchi chinensis) and rambutan (Nephelium lappaceum) cultivars grown in Hawaii. Journal of Food Composition and Analysis 19(6-7): 655-663.
- Pongsai P, Tongchitpakdee S, Fuongfuchat A, Chonhenchob V (2009) Effect of High Gas Permeable Materials on Quality and Shelf Life of Rambutan cv. Rong Rian. Kasetsart J 43(5): 275-281.
- Nakasone H, Paull R (1998) Tropical fruits. CAB International, Londres, pp. 45-75.
- Nimisha S, Kherwar D, Ajay K, Singh B, Usha K (2013) Molecular breeding to improve guava (Psidium guajava L.): Current status and future prospective. Scientia Horticulturae 164: 578-588.
- Laily N, Kusumaningtyas RW, Sukarti I, Rini MRDK (2015) The Potency of Guava (Psidium guajava L.) Leaves as a Functional Immunostimulatory Ingredient. Procedia Chemistry 14: 301-307.
- FOASTAT (2015) Food and agriculture organization of the United Nations statistics division. FOASTAT (2014) Food and agriculture organization of the United Nations statistics division.
- Kennedy M, List D, Lu Y, Foo L, Newman R, et al. (1999) Apple pomace and products derived from apple pomace: uses, composition and analysis. Analysis of Plant Waste Materials pp. 75-119.
- Waliaveetil EE, Ramesh SR (2003) Utilization of By-Products of Fruit and Vegetable Processing. In: Waliaveetil EE and Ramesh SR (Eds.), Handbook of Postharvest Technology. CRC Press, USA, pp. 819-844.
- Bhushan S, Kalia K, Sharma M, Singh B, Ahuja PS (2008) Processing of apple pomace for bioactive molecules. Crit Rev Biotechnol 28(4): 285-296.
- Carson KJ, Collins JL, Penfield MP (1994) Unrefined, dried apple pomace as a potential food ingredient. Journal of Food Science 59(6): 1213-1215.
- Henríquez C, Speisky H, Chiffelle I, Valenzuela T, Araya M, et al. (2010) Development of an ingredient containing apple peel, as a source of polyphenols and dietary fiber. J Food Sci 75(6): H172-H181.
- Wolfe KL, Liu RH (2003) Apple peels as a value-added food ingredient. J Agric Food Chem 51(6): 1676-1683.
- Łata B, Tomala K (2007) Apple Peel as a Contributor to Whole Fruit Quantity of Potentially Healthful Bioactive Compounds. Cultivar and Year Implication. J Agric Food Chem 55(26): 10795-10802.
- Singh A, Sabally K, Kubow S, Donnelly DJ, Gariepy Y, et al. (2011) Microwave-assisted extraction of phenolic antioxidants from potato peels. Molecules 16(3): 2218-2232.
- Boussetta N, Vorobiev E, Deloison V, Pochez F, Falcimaigne-Cordin A, et al. (2011) Valorisation of grape pomace by the extraction of phenolic antioxidants: Application of high voltage electrical discharges. Food Chem 128(2): 364-370.
- Aliakbarian B, Fathi A, Perego P, Dehghani F (2012) Extraction of antioxidants from winery wastes using subcritical water. Journal of Supercritical Fluids 65: 18-24.
- Kammerer D, Claus A, Schieber A, Carle R (2005) A novel process for the recovery of polyphenols from grape (Vitis vinifera L.) pomace. Journal of Food Science 70(2): C157-C163.
- Maier T, Schieber A, Kammerer DR, Carle R (2009) Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chemistry 112(3): 551-559.
- Kammerer D, Claus A, Carle R, Schieber A (2004) Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J Agric Food Chem 52(14): 4360-4367.
- Lu Y, Yeap Foo L (1999) The polyphenol constituents of grape pomace. Food Chemistry 65(1): 1-8.
- Martin-Carro N, Garcia-Alonso A, Goni I, Saura-Calixto F (1997) Nutritional and physiological properties of grape pomace as a potential food ingredient. American Journal of Enology and Viticulture 48(3): 328-332.
- Yu J, Ahmedna M (2013) Functional components of grape pomace: Their composition, biological properties and potential applications. International Journal of Food Science & Technology 48(2): 221-237.
- Fudholi A, Othman MY, Ruslan MH, Mat S, Sopian K, et al. (2013) Prospect and future of solar dryer for agricultural and marine product: perspective Malaysia. Proc of the 7th WSEAS Int Conf On Renewable Energy Sources (RES'13), pp. 141-149.
- Seveda MS, Rathore NS, Kumar V (2011) Solar tunnel dryer-a promising option for solar drying. Handbook of Renewable Energy Technology, pp. 289-320.
- Suman S, Khan MK, Pathak M (2015) Performance enhancement of solar collectors-A review. Renewable and Sustainable Energy Reviews 49: 192-210.
- Verma SK, Tiwari AK (2015) Progress of nanofluid application in solar collectors: A review. Energy Conversion and Management 100: 324-346.
- Jon CK, Kiang CS (2007) Food dehydration and developing countries. Food drying science and technology: Microbiology Chemistry Application 67.
- Chua K, Chou S (2003) Low-cost drying methods for developing countries. Trends in Food Science & Technology 14(12): 519-528.
- Sharma A, Chen CR, Lan NV (2009) Solar-energy drying systems: A review. Renewable and sustainable energy reviews 13(6-7): 1185-1210.
- Parikh DM (2014) Solids drying: basics and applications. Chemical Engineering 121(4): 42-45.
- Lewicki PP (2006) Design of hot air drying for better foods. Trends in Food Science & Technology 17(4): 153-163.
- Raghavan GSV, Orsat V (2007) Recent advances in drying of biomaterials for superior quality bioproducts. Asia-Pacific Journal of Chemical Engineering 2(1): 20-29.
- Monica AF, Cristina R (2008) Dehydration of Foods. Advances in Food Dehydration, CRC Press, USA, pp. 1-36.
- Brennan JG (2003) Fluidized-bed drying. Encyclopedia of food sciences and nutrition (2nd edn), In: Caballero B, Trugo L, Finglas PM (Eds.), Elsevier Science Ltd, pp. 1922-1929.
- Mujumdar AS, Devahastin S (2003) Applications for fluidized bed drying. Handbook of fluidization and fluid-particle systems, USA, pp. 469-484.
- Sloth J (2010) Method for improving spray drying equipment and product properties. Interceram 59: 193-197.
- Bhandari BR, Datta N, Howes T (1997) Problems associated with spray drying of sugar-rich foods. Drying Technology 15(2): 671-684.
- Muzaffar K, Nayik GA, Kumar P (2016) Stickiness problem associated with spray drying of sugar and acid rich foods: A mini review [2015]. Journal of Nutrition & Food Sciences s11(Suppl 11): 1-3.
- Phisut N (2012) Spray drying technique of fruit juice powder: some factors influencing the properties of product. International Food Research Journal 19(4): 1297-1306.
- Raghavan G, Orsat V (2008) Nonconventional Heating Sources during Drying. In: Raghavan G, Orsat V (Eds.), Advances in Food Dehydration, CRC Press, USA, pp. 401-422.
- Dev SRS, Geetha P, Orsat V, Gariepy Y, Raghavan GSV, et al. (2011) Effects of microwave-assisted hot air drying and conventional hot air drying on the drying kinetics, color, rehydration, and volatiles of Moringa oleifera. Drying Technology 29(12): 1452-1458.
- An K, Li H, Zhao D, Ding S, Tao H, et al. (2013) Effect of osmotic dehydration with pulsed vacuum on hot-air drying kinetics and quality attributes of cherry tomatoes. Drying Technology, 31(6): 698-706.
- Changrue V (2006) Hybrid (osmotic, microwave-vacuum) drying of strawberries and carrots, McGill University, Quebec, Canada.
- Therdthai N, Visalrakkij T (2012) Effect of osmotic dehydration on dielectric properties, microwave vacuum drying kinetics and quality of mangosteen. International Journal of Food Science & Technology 47(12): 2606-2612.
- Doymaz 1 (2012) Sun drying of seedless and seeded grapes. J Food Sci Technol 49(2): 214- 220.
- Kelebek H, Jourdes M, Selli S, Teissedre PL (2013) Comparative evaluation of the phenolic content and antioxidant capacity of sun- dried raisins. J Sci Food Agric 93(12): 2963-2972.
- Pandian N, Rajkumar P (2016) Sun and mechanical drying and study on drying rate kinetics of tamarind (Tamarindusindica L.) at different drying temperatures. Environment and Ecology 34(1A): 324-328.
- Ismail O, Figen AK, Pijkin S (2017) Open sun drying of green bean: influence of pretreatments on drying kinetics, colour and rehydration capacity. Heat and Mass Transfer 53(4): 1277-1288.
- Chowdhury M, Bala B, Haque M (2011) Energy and exergy analysis of the solar drying of jackfruit leather. Biosystems Engineering 110(2): 222-229.
- Fudholi A, Sopian K, Yazdi MH, Ruslan MH, Gabbasa M, et al. (2014) Performance analysis of solar drying system for red chili. Solar Energy 99: 47-54.
- Akhijani HS, Arabhosseini A, Kianmehr M (2016) Effective moisture diffusivity during hot air solar drying of tomato slices. Research in Agricultural Engineering 62(1): 15-23.
- Kulanthaisami S, Rajkumar P, Venkatachalam P, Subramanian P, Raghavan G, et al. (2010) Drying kinetics of tomato slices in solar cabinet dryer compared with open sun drying. Madras Agricultural Journal 97(7/9): 287-295.
- Sahin S, Sumnu G, Tunaboyu F (2013) Usage of solar-assisted spouted bed drier in drying of pea. Food & Bioproducts Processing 91(3): 271278.
- Saxena A, Maity T, Raju PS, Bawa AS (2012) Degradation kinetics of colour and total carotenoids in jackfruit (Artocarpus heterophyllus) bulb slices during hot air drying. Food & Bioprocess Technology 5(2): 672679.
- Demarchi SM, Ruiz NAQ, Concellon A, Giner SA (2013) Effect of temperature on hot-air drying rate and on retention of antioxidant capacity in apple leathers. Food and Bioproducts Processing 91(4): 310-318.
- Cano CM, Stringheta P, Ramos A, Cal Vidal J (2005) Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innovative Food Science & Emerging Technologies 6(4): 420-428.
- Quek SY, Chok NK, Swedlund P (2007) The physicochemical properties of spray-dried watermelon powders. Chemical Engineering and Processing: Process Intensification 46(5): 386-392.
- Goula AM, Adamopoulos KG, Kazakis NA (2004) Influence of spray drying conditions on tomato powder properties. Drying Technology 22(5): 1129-1151.
- Hatamipour MS, Mowla D (2006) Drying behaviour of maize and green peas immersed in fluidized bed of inert energy carrier particles. Food and Bioproducts Processing 84(3): 220-226.
- Senadeera W, Bhandari B, Young G, Wijesinghe B (2000) Physical properties and fluidization behavior of fresh green bean particulates during fluidized bed drying. Food and Bioproducts Processing 78(1): 43-47.
- Orikasa T, Koide S, Okamoto S, Imaizumi T, Muramatsu Y, et al. (2014) Impacts of hot air and vacuum drying on the quality attributes of kiwifruit slices. Journal of Food Engineering 125: 51-58.
- Wu L, Orikasa T, OgawaY, Tagawa A (2007) Vacuum drying characteristics of eggplants. Journal of Food Engineering 83(3): 422-429.
- Mahn A, Zamorano M, Barrientos H, Reyes A (2012) Optimization of a process to obtain selenium-enriched freeze-dried broccoli with high antioxidant properties. LWT-Food Science and Technology 47(2): 267-273.
- Voda A, Homan N, Witek M, Duijster A, van Dalen G, et al. (2012) The impact of freeze-drying on microstructure and rehydration properties of carrot. Food Research International 49(2): 687-693.
- Kumar D, Prasad S, Murthy GS (2014) Optimization of microwave- assisted hot air drying conditions of okra using response surface methodology. J Food Sci Technol 51(2): 221-232.
- Zhao D, An K, Ding S, Liu L, Xu Z, et al. (2014) Two-stage intermittent microwave coupled with hot-air drying of carrot slices: drying kinetics and physical quality. Food & Bioprocess Technology 7(8): 2308-2318.
- Workneh TS, Raghavan V, Gariepy Y (2011) Microwave assisted hot air ventilation drying of tomato slices. International Conference on Food Engineering and Biotechnology 9: 150-161.
- Askari GR, Emam DZ, Mousavi SM (2013) Heat and mass transfer in apple cubes in a microwave-assisted fluidized bed drier. Food and Bioproducts Processing 91(3): 207-215.
- Hu X, Kurian J, Gariepy Y, Raghavan V (2017) Optimization of microwave-assisted fluidized-bed drying of carrot slices. Drying Technology 35(10): 1234-1248.
- Hao J, Min Z, Arun SM, Rui XL (2014) Changes of microwave structure/dielectric properties during microwave freeze-drying process banana chips. International Journal of Food Science & Technology 49(4): 1142-1148.
- Duan X, Ren GY, Zhu WX (2012) Microwave freeze drying of apple slices based on the dielectric properties. Drying Technology 30(5): 535-541.
- Pu YY, Sun DW (2015) Vis-NIR hyperspectral imaging in visualizing moisture distribution of mango slices during microwave-vacuum drying. Food Chem 188: 271-278.
- Duan X, Huang LL, Wang MM, Qiao F, Fang CF, et al. (2015) Studies on the effects of microwave power and temperature control on the quality of whole lychee (Litchi chinensisSonn.) fruit during microwave vacuum drying. Journal of Food Processing and Preservation 39(4): 423-431.
- Borquez R, Melo D, Saavedra C (2015) Microwave-Vacuum drying of strawberries with automatic temperature control. Food and Bioprocess Technology 8(2): 266-276.
- Md Salim NS, Gariepy Y, Raghavan V (2017) Hot air drying and microwave-assisted hot air drying of broccoli stalk slices (Brassica oleracea L. Var Italica). Journal of Food Processing and Preservation 41(3): e12905.
- Corrêa J, Dev S, Gariepy Y, Raghavan G (2011) Drying of pineapple by microwave-vacuum with osmotic pretreatment. Drying Technology 29(13): 1556-1561.
- Sutar PP, Raghavan GVS, Gariepy Y, Prasad S, Trivedi A (2012) Optimization of osmotic dehydration of potato cubes under pulsed microwave vacuum environment in ternary solution. Drying Technology 30(13): 1449-1456.
- Patel J, Sutar P (2016) Acceleration of mass transfer rates in osmotic dehydration of elephant foot yam (Amorphophallus paeoniifolius) applying pulsed-microwave-vacuum. Innovative Food Science & Emerging Technologies 36: 201-211.
- Hardy G (2000) Nutraceuticals and functional foods: introduction and meaning. Nutrition 16(7-8): 688-689.
- Health Canada (2013) Policy paper-nutraceuticals/functional foods and health claims on foods, Vol 2016.
- Bravo L (1998) Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56(11): 317333.
- Baiano A, Bevilacqua L, Terracone C, Conto F, Del Nobile MA (2014) Single and interactive effects of process variables on microwave- assisted and conventional extractions of antioxidants from vegetable solid wastes. Journal of Food Engineering 120(1): 135 145.
- Ajila CM, Brar SK, Verma M, Tyagi RD, Godbout S, et al. (2011) Extraction and analysis of polyphenols: Recent trends. Crit Rev Biotechnol 31(3): 227-249.
- Ballard TS, Mallikarjunan P, Zhou K, O'Keefe S (2010) Microwave- assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chemistry 120(4): 1185-1192.
- Beveridge T (1997) Juice extraction from apples and other fruits and vegetables. Crit Rev Food Sci Nutr 37(5): 449-469.
- Waldron KW (2007) Handbook of waste management and co-product recovery in food processing. In: Waldron KW (Ed.), Handbook of waste management and co-product recovery in food processing (1st edn), Wood head Publishing, UK, pp. 1-680.
- Escarpa A, Gonzalez MC (2008) An overview of analytical chemistry of phenolic compounds in foods. Crit Rev Anal Chem 31(2): 57-139.
- Galanakis CM (2012) Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends in Food Science and Technology 26(2): 68-87.
- Parmar I, Sharma S, Rupasinghe HV (2015) Optimization of β-cyclodextrin-based flavonol extraction from apple pomace using response surface methodology. J Food Sci Technol 52(4): 22022210.
- Leão DP, Botelho BG, Oliveira LS, Franca AS (2017) Potential of pequi (Caryocar brasiliense Camb.) peels as sources of highly esterified pectins obtained by microwave assisted extraction. LWT- Food Science and Technology 87: 575-580.
- Benítez V, Mollá E, Martím-Cabrejas MA, Aguilera Y, LÓpez-Andréu FJ, et al. (2011) Characterization of industrial onion wastes (Allium cepa L.): Dietary fibre and bioactive compounds. Plant Foods for Hum Nutr 66(1): 48-57.
- Schieber A, Hilt P, EndreR HU, Rentschler C, Carle R, et al. (2003) A new process for the combined recovery of pectin and phenolic compounds from apple pomace. Innovative Food Science & Emerging Technologies 4(1): 99-107.
- Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B (2008) Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innovative Food Science and Emerging Technologies 9(1): 85-91.
- Ghafoor K, Choi YH, Jeon JY, Jo IH (2009) Optimization of ultrasound- assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J Agric Food Chem 57(11): 4988-4994.
- Li Y, Han L, Ma R, Xu X, Zhao C, et al. (2012) Effect of energy density and citric acid concentration on anthocyanins yield and solution temperature of grape peel in microwave-assisted extraction process. Journal of Food Engineering 109(2): 274-280.
- Liazid A, Guerrero R, Canto E, Palma M, Barroso C (2011) Microwave assisted extraction of anthocyanins from grape skins. Food Chemistry 124(3): 1238-1243.
- Luque-Rodnguez JM, Luque de Castro MD, Perez-Juan P (2007) Dynamic superheated liquid extraction of anthocyanins and other phenolics from red grape skins of winemaking residues. Bioresour Technol 98(14): 2705-2713.
- Adil IH, £etin HI, Yener ME, Bayindirli A (2007) Subcritical (carbondioxide+ethanol) extraction of polyphenols from apple and peach pomaces, and determination of the antioxidant activities of the extracts. Journal of Supercritical Fluids 43(1): 55-63.
- Koiodziejczyk K, Markowski J, Kosmala M, Krol B, Piocharski W, et al. (2007) Apple pomace as a potential source of nutraceutical products. Pol J Food Nutr Sci 57(4): 291-295.
- Krishnaswamy K, Orsat V, Gariepy Y, Thangavel K (2013) Optimization of microwave-assisted extraction of phenolic antioxidants from grape seeds (Vitis vinifera). Food and Bioprocess Technology 6(2): 441-455.
- Pedroza MA, Amendola D, Maggi L, Zalacain A, De Faveri, et al. (2015) Microwave-assisted extraction of phenolic compounds from dried waste grape skins. International journal of food engineering 11(3): 359-370.
- Pan X, Niu G, Liu H (2003) Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chemical Engineering and Processing 42(2): 129-133.
- Perva-Uzunali A, Škerget M, Knez (2004) Isolation of active ingredients from green tea (Fanning Bellas, China) Proceedings of the 16th International Congress of Chemical and Process Engineering-CHISA.
- Serdar G, Demir E, Sokmen M (2017) Recycling of tea waste: Simple and effective separation of caffeine and catechins by microwave assisted extraction (MAE). International Journal of Secondary Metabolite 4(2).
- Sökmen M, Demir E, Alomar SY (2018) Optimization of sequential supercritical fluid extraction (SFE) of caffeine and catechins from green tea. The Journal of Supercritical Fluids 133(1): 171-176.
- Baysal T, Ersus S, Starmans DAJ (2000) Supercritical CO2 extraction of p-carotene and lycopene from tomato paste waste. J Agric Food Chem 48(11): 5507-5511.
- Ho K, Ferruzzi M, Liceaga A, San Martm-Gonzalez M (2015) Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT- Food Science and Technology 62(1): 160-168.
- Kehili M, Kammlott M, Choura S, Zammel A, Zetzl C, et al. (2017) Supercritical CO2 extraction and antioxidant activity of lycopene and p-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food and Bioproducts Processing 102: 340-349.
- Galanakis CM, Tornberg E, Gekas V (2010a) Clarification of high- added value products from olive mill wastewater. Journal of Food Engineering 99(2): 190-197.
- Galanakis CM, Tornberg E, Gekas V (2010b) The effect of heat processing on the functional properties of pectin contained in olive mill wastewater. LWT-Food Science and Technology 43(7): 10011008.
- Singh A, Nair GR, Liplap P, Gariepy Y, Orsat V, et al. (2014) Effect of Dielectric Properties of a Solvent-Water Mixture Used in Microwave- Assisted Extraction of Antioxidants from Potato Peels. Antioxidants 3(1): 99-113.
- Luque de Castro MD, Garcia-Ayuso LE (1998) Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Analytica Chimica Acta 369(1-2): 1-10.
- Sporring S, B0wadt S, Svensmark B, Bjorklund E (2005) Comprehensive comparison of classic Soxhlet extraction with Soxtec extraction, ultrasonication extraction, supercritical fluid extraction, microwave assisted extraction and accelerated solvent extraction for the determination of polychlorinated biphenyls in soil. Journal of Chromatography A 1090(1-2): 1-9.
- Lafka TI, Lazou AE, Sinanoglou VJ, Lazos ES (2011) Phenolic and antioxidant potential of olive oil mill wastes. Food Chemistry 125(1): 92-98.
- Lafka TI, Sinanoglou V, Lazos ES (2007) On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chemistry 104(3): 1206-1214.
- Koubala BB, Mbome LI, Kansci G, Tchouanguep Mbiapo F, Crepeau MJ, et al. (2008) Physicochemical properties of pectins from ambarella peels (Spondias cytherea) obtained using different extraction conditions. Food Chemistry 106(3): 1202-1207.
- Chen T, Sun X, Xiao W, Liu X, Zhang W, et al. (2010) Optimization of microwave-assisted extraction of solanesol from potato leaves and stems. Medicinal Chemistry Research 19(8): 732-742.
- Dang YY, Zhang H, Xiu ZL (2013) Microwave-assisted aqueous two-phase extraction of phenolics from grape (Vitis vinifera) seed. Journal of Chemical Technology and Biotechnology 89(10): 15761581.
- Nemes SM, Orsat V (2009) Microwave-assisted extraction of secoisolariciresinol diglucoside-method development. Food Bioprocess Technol pp. 1-9.
- Oreopoulou V, Tzia C (2007) Utilization of plant by-products for the recovery of proteins, dietary fibers, antioxidants, and colorants. Utilization of By-Products and Treatment of Waste in the Food Industry pp. 209-232.
- Périno-Issartier S, Zill e H, Abert-Vian M, Chemat F (2011) Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food and Bioprocess Technology 4(6): 1020-1028.
- Wang S, Chen F, Wu J, Wang Z, Liao X, et al. (2007) Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. Journal of Food Engineering 78(2): 693-700.
- Routray W, Orsat V (2012) Microwave-Assisted extraction of flavonoids: A review. Food & Bioprocess Technology 5(2): 409-424.
- Herrero M, Mendiola JA, Cifuentes A, Ibanez E (2010) Supercritical fluid extraction: Recent advances and applications. J Chromatogr A 1217(16): 2495-2511.
- Bernardo-Gil MG, Roque R, Roseiro LB, Duarte LC, Grno F, et al. (2011) Supercritical extraction of carob kibbles (Ceratonia siliqua L.). Journal of Supercritical Fluids 59: 36-42.
- Giannuzzo AN, Boggetti HJ, Nazareno MA, Mishima HT (2003) Supercritical fluid extraction of naringin from the peel of Citrus paradisi. Phytochem Anal 14(4): 221-223.
- Crea R (2002) Method of obtaining a hydroxytyrosol-rich composition from vegetation water
- Santos DN, De Souza LL, Ferreira NJ, De Oliveira AL (2011) Process variables study on supercritical CO2 extraction of Brazilian cherry seeds (Eugenia uniflora L.) rich in bioactive volatile. Food process engineering in a changing world, pp. 1669-1670.
- Cavero S, Garria-Risco MR, Marin FR, Jaime L, Santoyo S, et al. (2006) Supercritical fluid extraction of antioxidant compounds from oregano. Chemical and functional characterization via LC-MS and in vitro assays. Journal of Supercritical Fluids 38(1): 62-69.
- Farfas-Campomanes AM, Rostagno MA, Meireles MAA (2013) Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. Journal of Supercritical Fluids 77: 70-78.
- Ghafoor K, Park J, Choi YH (2010) Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B. ) peel by using response surface methodology. Innovative Food Science and Emerging Technologies 11(3): 485-490.
- Adam F, Abert Vian M, Peltier G, Chemat F (2012) Solvent-free ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresour Technol 114: 457-465.
- Chemat F, Zill EH, Khan MK (2011) Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrasonics Sonochemistry 18(4): 813-835.
- Chen Y, Luo H, Gao A, Zhu M (2011) Ultrasound-assisted extraction of polysaccharides from litchi (Litchi chinensis Sonn.) seed by response surface methodology and their structural characteristics. Innovative Food Science and Emerging Technologies 12(3): 305309.
- Kaewkiew J, Nabnean S, Janjai S (2012) Experimental investigation of the performance of a large-scale greenhouse type solar dryer for drying chilli in Thailand. Procedia Engineering 32: 433-439.
- Nemes SM, Orsat V (2011) Microwave-Assisted extraction of secoisolariciresinol diglucoside-method development. Food and Bioprocess Technology 4(7): 1219-1227.
- Romdhane M, Gourdon C (2002) Investigation in solid-liquid extraction: Influence of ultrasound. Chemical Engineering Journal 87(1): 11-19.
- Patist A, Bates D (2008) Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innovative Food Science and Emerging Technologies 9(2): 147-154.
- (2015) World population prospects: The 2015 revision, key findings and advance tables, P.D. department of economic and social affairs, New York, USA.
- Parry A, James K, LeRoux S (2015) Strategies to achieve economic and environmental gains by reducing food waste. Banbury, England.
© 2017 Nora Salina Md Salim, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






