- Submissions

Full Text
Modern Concepts & Developments in Agronomy
Dimethyl Ether and Dibutyl Ether Produced from Biogas and Biomass and Industrial Waste Gas
Johann Gruber Schmidt*
Technical University Vienna, Australia
*Corresponding author: Johann Gruber-Schmidt, Technical University Vienna, Theresiengasse 47, A 1180 Vienna, Australia
Submission: February 08, 2018;Published: April 13, 2018

ISSN: 2637-7659 Volume2 Issue1
Introduction
At the begin of the 20th century C. Weizmann studied methods for substitution of petroleum, the most important fuel at this time beside coal. His famous work led to the patent about the ABE process [1], producing acetone by clostridium acetobutylicum and beside acetone, butanol (C4 alcohol) and ethanol (C2 alcohol) are created. (Historical: acetone could be used in military applications). The ideas [1] were very fruitful, but the world wars and the huge amount crude oil in Texas started a new period of economic development and growth of the world trade and business. At the end of the 20th century we recognized the limitation of the raw materials. Additional the Diesel technology reached a satisfied region in development the pollution of the exhaust gas. 2005 VOLVO started a project replacing fossil Diesel by Dimethyl ether [2].
This was so successful and it was the prolongation of a development done in 2008 by VOLVO [2]. At this time no heavy truck company was interested. At the end of the project VOLVO entered with the DME trucks successful the US market. The next company testing DME was MACK TRUCK followed two years later FORD. This was the birth of a new market. DME has the advantage to be monomolecular fuel, which can be easy supplied to existing and new Diesel engines. Using the known common rail for DME injected into the combustion chamber of engines it changes the phase from liquid to gas. This important property leads to a dramatic reduction of exhaust gas pollution in carbon monoxide, nitrogen oxide and soot, fine particles.
Agriculture and DME
In the agriculture we have a huge amount biogenic waste material. The wet waste biogenic material we be used in fermentation processes producing biogas (gas mixture of methane and carbon dioxide) [4]. The solid biogenic waste material will be used in producing a very weak syngas (gas mixture of carbon monoxide, hydrogen and carbon dioxide) by thermo chemical conversion [5]. But this weak gas is good enough to support the process of steam gasification of biogenic solid fine mass. Using the char, fine melted biogenic mass we can supply steam gasification combined with biogas supplying methane gasification producing the known water gas and generator gas. This syngas has high caloric heat value enabling to support the production of Dimethyl ether, over methanol synthesis and dehydration, or direct synthesis. The conversion efficiency is given in the range of 35% up to 75%. The off gas of the Dimethyl ether synthesis is recycled and reused again. The synthesis processes are operating at a pressure of 50 bar and mean temperature of 250 °C supported by catalysts like CuO-ZnO-Al2O3 (acid catalysts) and H-ZSM5 (dehydration). This synthesis is very easy and simple to be realized and can be applied to all known agricultural processes converting biomass and biogas to liquid fuel. The processes can be enlarged by using waste (solid, liquid). Burning waste leads to huge problems in cleaning up the exhaust gases to reduce pollution. Thermo chemical conversion and fermentation do not lead to exhaust gases responsible for pollution of the environment.
Figure 1: The combination of fermentation and thermochemical conversion with steam, methane gasification.
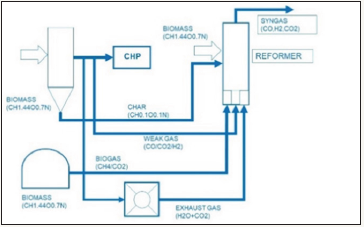
Waste separated, recycled and refined is an additional supplement of biomass and helps to save fresh new biomass. We again have learned that also fresh biomass is limited and therefore waste replaces the needed fresh biomass and enables “waste to liquid fuel” [6] (Figure 1). The handling of DME is very easy, cheap and safe. But again we have to store it at pressure ~6 bars at least. Some are therefore speaking from a biogenic LPG, called DME, replacing fossil LPG (liquid propane gas) (Figure 2).
Figure 2:
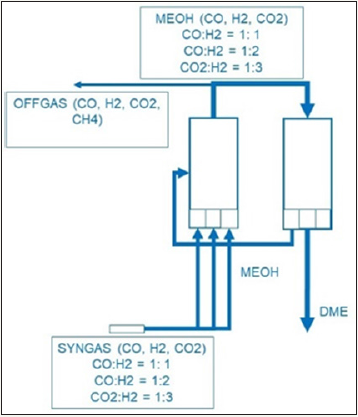
DBE
Now the very important ideas of C. Weizmann are taking place. Using DME we can convert DME in very easy way to ethanol. This conversion of DME to ethanol is supported by carbonylation (carbon monoxide) and hydrogenation (hydrogen) over the intermediate product methyl acetate (CH3COOCH3). The conversion process of DME to ethanol is so simple and cheap, that in the next step ethanol can be converted to 1-butanol (sometimes also called n-butanol). Let us remember: the fermentation process with bacteria C… A… lead to a combination of acetone, butanol and ethanol, well known as ABE process invented by C. Weizmann [1]. The conversion of DME enables and supports the conversion to ethanol and 1-butanol.
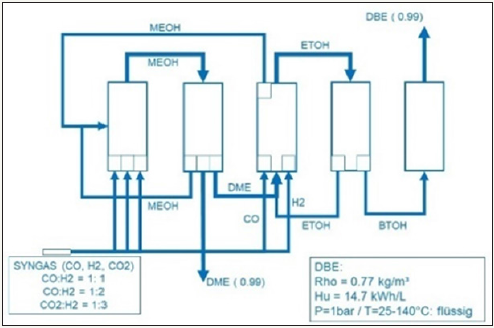
The conversion efficiency is in the range of 50% up to 75%. At the end 1-butanol is in our main interest. Polishing 1-butanol by dehydration to dibutyl ether leads to very interesting properties: boiling temperature Tb = 141 °C under environment pressure p~ 1bar, flash temperature Tf = 25°C, molar weight MZ =130g/mol. The thermodynamic properties caloric heat value Hu=14.7k J/l = 11.2kJ/kg, density r=770kg/m³. The chemical formula of dibutyl ether is C4H18O. The properties of Dimethyl ether C2H6O have the following properties: boiling temperature Tb = -24 °C under environment pressure p~ 1bar, flash temperature Tf = -41°C, molar weight MZ= 46g/mol. The thermodynamic properties caloric heat value Hu=8.3k J/L =5.56kJ/kg, density r=650kg/m³. Fossil Diesel has heat caloric value between DME (Dimethyl ether) and DBE (Dibutyl ether) (Figure 3)
Figure 3: Conversion of DME to ethanol, 1- butanol and DBE.
Waste Gas from Industry
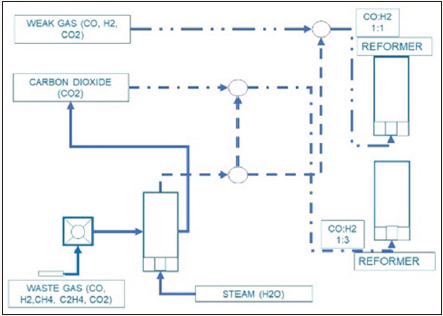
In the next step we can combine the biogenic driven processes with industrial processes. Industrial processes like coke oven gas, or blast furnace gas, or landfill gas, or shale gas, instead of flaring we can use it. The combination of biogas, weak gas from biomass, and waste gas, enable to produce hydrogen with chemical looping supported by metal catalysis, in cheap and easy way. This hydrogen generated from steam is used to refine and polish the gas mixture for the methanol synthesis. More than, this simple step enables to use more carbon dioxide than produced by the process of hydrogen generation itself, working as a strong carbon dioxide sink. The combination industrial waste gas, biogas, and weak gas form gasification lead to the integral and flexible preparation of the syngas mixtures used for the methanol synthesis (CO: H2 = 1:2; CO2: H2 = 1:3) or dimethyl ether synthesis (CO: H2 = 1:1). This combination is supported by a cheap hydrogen production from water by metal and metal oxides, known as chemical looping, and the steam reforming of carbon hydrates (C1...C4) to carbon monoxide and hydrogen, are the additional basic properties to enlarge the production of methanol and dimethyl ether (Figure 4).
Figure 4: Gas refining, gas preparation to high quality syngas.
Conclusion
In this short article we have remembered the possibility and benefits in substitution of fossil Diesel by Dimethyl ether. This is well known and a very successful application with high economic benefits- Dimethyl ether can be produced from biomass and biogas, and in combination of both processes. Hence possessing Dimethyl ether we can produce 1-Butanol and by dehydration Dibutyl ether in a very efficient way. The properties and the benefits of dibutyl ether are shown in this article. The aim of reducing the need of fresh new biomass from wood and agriculture, leads directly to a combination of the biogenic processes with industrial processes and waste gas from industrial processes is shown.
Let us do a look into the future: with these new green technologies we develop technologies we need colonizing our planets moon and mars, to survive. But we should be aware we need new very high energetic resources entering space and travelling in a cheap and easy way to our next planets, leaving behind the fossil age.
References
- Patent GB (1909) The “Weizmann Process”. Oxford University Press, India.
- (2013) VOLVO Trucks, DME Fact Sheets.
- Saxmann (2016) DME for Heavy-Duty Trucking, VOLVO Trucks.
- Rosanto MA (2017) Managing Biogas Plants: A Practical Guide (Green Chemistry and Chemical Engineering), CRC Press, USA.
- Heidenreich S (2016) Advanced Biomass Gasification: New Concepts for Efficiency Increase and Product Flexibility, Academic Press, USA.
- Nick Kanellopoulos (2015) Small-Scale Gas to Liquid Fuel Synthesis, CRC Press, USA.
© 2018 Johann Gruber-Schmidt. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






