- Submissions

Full Text
Modern Applications in Pharmacy & Pharmacology
Medicinal Herbs, Strong Source of Antioxidant in Aquaculture: A Mini Review
Mohammad Mohiseni*
Department of Fisheries, Behbahan Khatam Alanbia University of Technology, Iran
*Corresponding author: Mohammad Mohiseni, Assistant Professor of Fisheries, Department of Fisheries, Behbahan Khatam Alanbia University of Technology, Iran
Submission: September 12, 2017; Published: November 10, 2017

ISSN 2637-7756Volume1 Issue1
Abstract
As a result of the severe decline in major marine and freshwater fish stocks, aquaculture has been greatly developed throughout the world to provide global fish demands. Not surprisingly, the estimated risk of fish production failure was elevated due to the intensification of fish culture, amplification of environmental stressors, water quality deterioration, fish disease outbreak and finally high rates of fish mortality. Aqua culturists inevitably turned to antioxidant feed additives in order to overcome problems regarding the elevation of fish physiological fitness. Various natural and chemical additives have been successfully used in fish diet. However, the utilization rate of chemical supplements is limited because of their high price and also potential toxicity for fish in some cases. Recently, the use of plant products in aquaculture presents an increasing importance or significance. Plant products contain a broad range of active molecules such as flavonoids, terpenoids, ascorbic acid, tocopherols and phenolic compounds which can protect cells against xenobiotics and environmental stressors because of having strong antioxidant properties. This paper presents brief information about the potential advantages of applying herbal medicine as an antioxidant in aquaculture.
Keywords: Herbs; Fish health; Stress; Free radicals
Introduction
Oxidative stress can damage various important biological molecules. The most significant targets of cellular injury are proteins and DNA. Another target of ROS is the lipids of cell membranes [1]. Lipids attacked by free radicals subsequently convert to lipid peroxidation. This metabolite is toxic and is capable to damage most cells and tissues [2,3]. There are evidences for significant contribution of lipid per oxidation to the progress of atherosclerosis, stroke and myocardial infarction (Priscilla & Prince, 2009). They also could exert a cancer promotional effect when originated from dying cells [4].
Traditionally, herbal medicines or medicinal plants are thought to be a major source of treatment in human or animal disease [5]. Having a lower price and greater accuracy with the lower side effects compared to chemical additives, encouraged aquaculturist to increase the use of herbal products for promoting animal health and production [6,7]. Generally, some herbal plants have different functions due to the presence of numerous active compounds. Antioxidants are one of the main parts of the herbal structure with a high content of bioactive compounds which shown to have the ability to inhibit ROS generation and to scavenge free radicals [8]. Plants are a potential source for providing natural antioxidants. Carotenoids, flavonoids, tocopherols, cinnamic acid, folic acid, ascorbic acid, etc. are the most prominent antioxidants produced by plants [9]. In this paper, three main groups of antioxidants were briefly presented and some exemplifications of successful use of medicinal herbs in aquaculture are discussed.
Carotenoids
Carotenoids are organic pigments produced mostly by plants which can be found in flowers and leafy parts of Herbs, [10] varying in color from yellow, orange and red. Carotenoids are strong antioxidants with scavenging potential of free radicals. They provide protection against oxidative damage to cells. Herbs that are rich in carotenoids may have an immune stimulant function. They also possess slight anti-inflammatory action and can reduce the risk of heart disease and cancer [11]. Lycopene, is a red colored carotenoid found in many fruits, vegetables and herbs. Having a high number of conjugated dienes, recommends lycopene as the most potent antioxidant, with radical quenching ability twice more than β-carotene and 10 times higher than those recorded from α-tocopherol [12]. In agreements, lycopene plays a crucial role in preventing from serious diseases such as cancer, hypertension and cardiovascular disease and lead to improve immune function and normal metabolic reactions.
Carotenoids are known to have a various function in fish. Fish do not have the ability to synthesize carotenoids, they must obtain these compounds from their diets [13]. Carotenoids are usually transferred and deposited in different tissues including skin (as xanthophyll esters in the chromatophore), muscle (astaxanthin especially in salmonids), the ovaries (carotenes during the reproduction in developing eggs) and liver [10]. Carotenoids transportation and deposition are carried in the circulatory system in association with lipoproteins. Fish eggs are one the main deposition site of the carotenoids. Vitellogenin, the precursor of the egg yolk lipovitelin, was suggested to function in carotenoids transportation, mainly Asthaxanthins, from the muscle to the ovary during spawning migration in salmonids. Carotenoids are believed that to have a fundamental role in successful egg fertilization, egg pigmentation and embryonic development [14].
Carotenoids are also the important source of vitamin A. Astaxanthin, canthaxanthin and zeaxaxanthin were found to be precursors of both vitamin A1 and A2 in some fishes. Liver and intestinal wall are active sites of carotenoids transformation into the vitamin A [13,15]. The rate of conversion is dependent on the vitamin A status and seems vary among different species [16].
Carotenoids are considered as non-enzymatic group of the cell’s antioxidant system and provide two mechanisms to protect against oxidative damage: 1) quenching of singlet oxygen and 2) scavenging of radicals [17]. Therefore, beside of the different functions including skin and tissue pigmentation [16] and growth promotor [18], carotenoids have multifunction as antioxidant. A relationship between dietary carotenoids concentration and antioxidant status in liver and muscle was reported in Atlantic salmon [13] and rainbow trout [19].
Flavonoids
Flavonoids are a remarkably large group of natural phenolic compounds which are found universally in plants. To date, over 6000 chemical constituents have been isolated and identified as flavonoids [6]. Flavonoids are secondary metabolite of plants with polyphenolic structure. Synthesize of the flavonoids are take place by the polypropanoid pathway and phenylalanine is the startup molecule [20]. These bioactive compounds are confirmed to be plant, flower, leaf and fruits pigments. They can be derived from various vegetables, nuts, seeds, grains and herbs [4]. Flavonoids are naturally occurred in living cells as glycosides and may break down to their constituent, aglycone and sugar by enzymes treatment [21]. As a result of marked antioxidant characteristics of flavonoids, they have been considered as aprimary antioxidant which can act as free radical acceptor and chain breaker [22-24]. Moreover, they can render toxic metal ions catalytically inactive by chelation. This process has a critical role in detoxification of metals within the cells [21].
Generally, the antioxidant activities of flavonoids are mediated by the subsequent mechanisms:
a) Scavenging free radicals.
b) Suppressing ROS production via enzymes inhibition or Indentation is needed metals involved in ROS formation.
c) Protecting through antioxidant defenses [20].
Recently phenolics and flavonoids have been introduced as great antioxidants and several reports have emphasized on their higher effectiveness over well-known antioxidants, vitamin C, E and carotenoids [6,7,25]. Clove, sage, garlic, ginger, rosemary and thyme contain a considerable amount of flavonoids [5,21,26].
Tocopherols
Tocopherols are monophenolic antioxidants that can be widely found in nature. They include eight distinct compounds and related to two families, tocols and tocotrienols [21]. Tocopherols are widely produced and used as food antioxidant [21]. Depending on the number and location of methyl groups attached to the chromane rings, they have the prefix α, β, γ or δ [27,28]. α-tocopherol (wellknown as vitamin E) is the most efficient member of this family. Vitamin E is one of the most effective free radical scavenging compounds that has a vital role in the normal structuring of cellular membrane [29]. In other words, the stability and function of cell membrane is completely dependent on the absorption capacity of the membrane when an extra amount of vitamin is available [27]. Vegetables and herbs, cereals and their products, seeds and nuts are the richest source of tocopherols [21]. The role of vitamin E in fish physiology and normal cellular function is well understood [3,30,31]. This vitamin is an essential nutrient required to maintain flesh quality, immunity, prevention from red blood haemolysis, the permeability of capillaries and heart muscle safety. As poly unsaturated fatty acids are the main and vital parts of the lipid structures in the fish body, the main function of vitamin E is to protect them against oxidation [32].
Antioxidant medicinal herb in aquaculture
Amongst the numerous herbal medicines, some of them have been successfully used in the fish diet. Medicinal herbs were used in aquaculture for different purposes. For example, herbs showed strong potential in a wide range of viral, bacterial or fungal disease [33-36]. Because of the fair price and strong effects of herbs, they have rapidly becoming a safe alternative for antibiotics and chemical drugs in aquaculture [8]. Elevation in growth performance and feed utilization of medicinal herbs are two important approaches in aquaculture. Active components within the herbs are believed to increase nutrient digestibility, absorption and assimilation capacity. Furthermore, these bioactive compounds can improve digestive enzymes secretion and maintain healthy intestinal microflora [25,37-40].
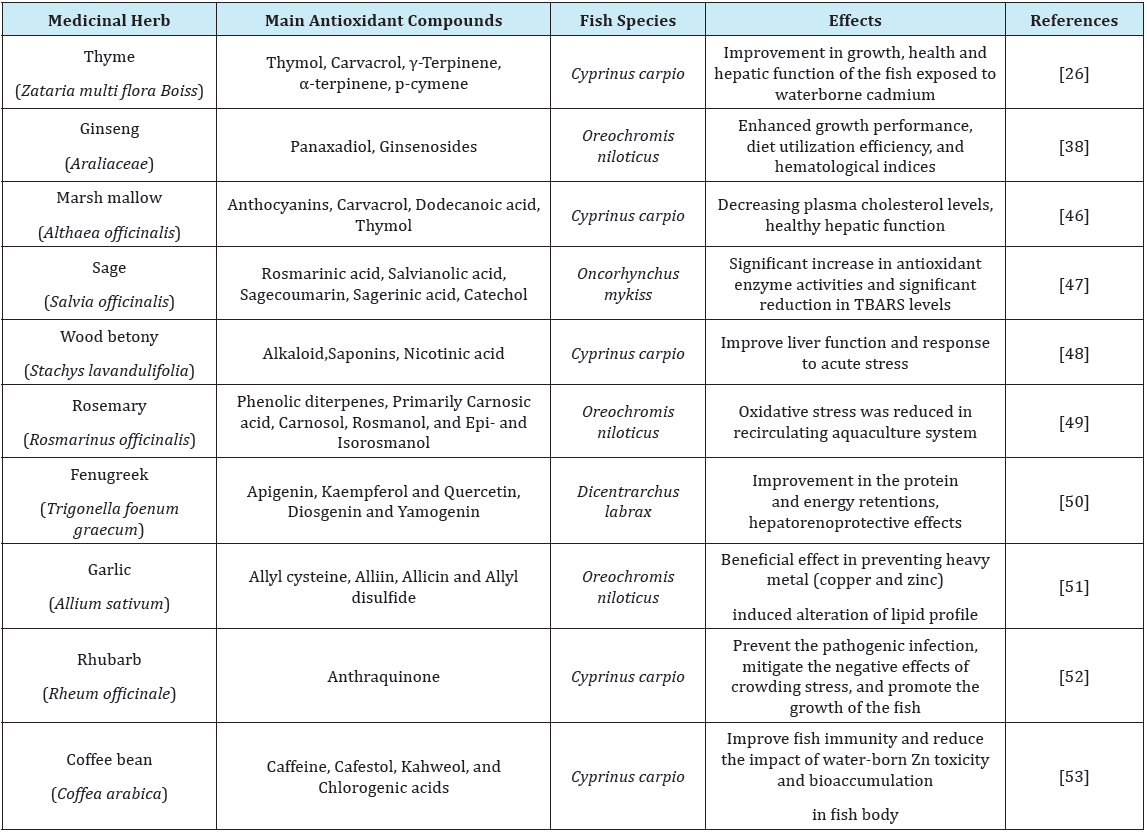
The use of medicinal herbs as an antioxidant source is a new approach in aquaculture. Intensive aqua farming systems have exposed the fish to several forms of stressors, as chemical, biological and physical disturbances, which can lead to severe alteration of the fish physiological condition. If these stressors surpass the fish biological threshold, the homeostasis will be disrupted and consequently stress outcomes will threat the animal’s health [7]. Plants contain a wide variety of biochemical compounds with antioxidant properties. These can help organisms to cope with oxidative stress caused by environmental stressors, hence, keep the fish physiological fitness [41]. The amelioration of negative impacts of environmental stressors by medicinal herb treatment in different fish species is represented in Table 1.
One of the most interesting characteristic of herbal antioxidants is related to their capacity to interact with other antioxidants. In this regards, antioxidants act synergistically, offering more protection than single administration. Antioxidants derived from plants, as polyphenols and flavonoids, may increase the effectiveness of vitamins [42] and may have compensatory effects when the vitamin levels are low [43]. In agreement, Antache et al. [44] stated that administration of thyme in combination with vitamin E had a synergistic effect on growth performance and reducing lipid peroxidation in Nile tilapia Mekkawy et al. [45] were also emphasized on the probable additive or synergistic effects of tomato paste carotenoids and vitamin E protecting from harmful effects of cadmium in Nile tilapia (Table 1)[46-53].
Table 1: Some medicinal herbs with antioxidant properties and their successful application for control of stress in different fish species
Conclusion and Perspectives
Medicinal herbs represent a potential resource in aquaculture with a large application field. They can be used as appetite stimulator, antimicrobial and antiviral effectors, immune stimulant and growth promoter. Due to their bioactive ingredients, e.g. polyphenols, flavonoids, tocopherols and essential oils, medicinal herbs present antioxidant properties and can be used as anti-stress remedy in aquaculture. This approach can reduce the costs and side effects of synthetic or chemical products. Medicinal herbs are also eco-friendly compounds and hence will not affect the environment. Despite to the presence of numerous antioxidant compounds in the most medicinal plants, but a few of them have been considered as a feed additive in fish diet. It seems that our knowledge about their application to aquatic animal health and production is still limited and further studies on this subject are needed [54-56].
References
- Gate L, Paul J, Ba GN, Tew K, Tapiero H (1999) Oxidative stress induced in pathologies: the role of antioxidants. Biomed Pharmacother 53(4): 169-180.
- Okawa M, Kinjo J, Nohara T, ONO M (2001) DPPH (1, 1-diphenyl-2- picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol Pharm Bull 24(10): 1202-1205.
- Mohiseni M, Asayesh S, Shafiee BS, Mohseni F, Moradi N, et al. (2016) Biochemical Alteration Induced by Cadmium and Lead in Common Carp via an Experimental Food Chain. Iranian Journal of Toxicology 10(4): 25- 32.
- Kandaswami C, Middleton E (1994) Free radical scavenging and antioxidant activity of plant flavonoids Free radicals in diagnostic medicine. Springer, pp. 351-376.
- O’Hara M, Kiefer D, Farrell K, Kemper K (1998) A review of 12 commonly used medicinal herbs. Arch Fam Med 7(6): 523-536.
- Ghasemzadeh A, Ghasemzadeh N (2011) Flavonoids and phenolic acids: Role and biochemical activity in plants and human. Journal of Medicinal Plants Research 5(31): 6697-6703.
- Syahidah A, Saad C, Daud H, Abdelhadi Y (2015) Status and potential of herbal applications in aquaculture: A review. Iranian Journal of Fisheries Sciences 14(1): 27-44.
- Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquaculture International 18(3): 403-414.
- Ghasemzadeh A, Jaafar HZ, Rahmat A (2010) Antioxidant activities total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 15(6): 4324-4333.
- Tacon AG (1981) Speculative review of possible carotenoid function in fish. The Progressive Fish-Culturist 43(4): 205-208.
- Craig WJ (1999) Health-promoting properties of common herbs. Am J Clin Nutr 70(3): 491s-499s.
- Sahin K, Yazlak H, Orhan C, Tuzcu M, Akdemir F, et al. (2014) Aquaculture 418-419: 132-138.
- Christiansen R, Glette J, ø Lie, Torrissen O, Waagbø R (1995) Antioxidant status and immunity in Atlantic salmon, Salmo salar L., fed semi‐purified diets with and without astaxanthin supplementation. Journal of Fish Diseases 18(4): 317-328.
- Lubzens E, Lissauer L, Levavi Sivan B, Avarre JC, Sammar M (2003) Carotenoid and retinoid transport to fish oocytes and eggs: what is the role of retinol binding protein? Mol Aspects Med 24(6): 441-457.
- Kaisuyama M, Matsuno T (1988) Carotenoid and vitamin A, and metabolism of carotenoids, β-carotene, canthaxanthin, astaxanthin, zeaxanthin, lutein and tunaxanthin in tilapia Tilapia nilotica. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 90(1): 131-139.
- Bendich A, Olson JA (1989) Biological actions of carotenoids. The FASEB J 3(8): 1927-1932.
- Wang YJ, Chien YH, Pan CH (2006) Effects of dietary supplementation of carotenoids on survival, growth, pigmentation, and antioxidant capacity of characins, Hyphessobrycon callistus. Aquaculture 261(2): 641-648.
- Kalinowski C, Robaina L, Fernandez PH, Schuchardt D, Izquierdo M (2005) Effect of different carotenoid sources and their dietary levels on red porgy (Pagrus ) growth and skin colour. Aquaculture 244(1-4): 223- 231.
- Page G, Russell P, Davies S (2005) Dietary carotenoid pigment supplementation influences hepatic lipid and mucopolysaccharide levels in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol B Biochem Mol Biol 142(4): 398-402.
- Cotelle N (2001) Role of flavonoids in oxidative stress. Curr Top Med Chem 1(6): 569-590.
- Shahidi F, Janitha P, Wanasundara P (1992) Phenolic antioxidants. Critical reviews in food science and nutrition 32(1): 67-103.
- Rice Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends in Plant Science 2(4): 152-159.
- Škerget M, Kotnik P, Hadolin M, Hraš AR, Simonič M, et al. (2005) Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food chemistry 89(2): 191-198.
- Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri industrial by-products: Antioxidant activity, occurrence, and potential uses. Food chemistry 99(1): 191-203.
- Mohiseni M, Sepidnameh M, Bagheri D, Banaee M, Nematdust HB (2017) Comparative effects of Shirazi thyme and vitamin E on some growth and plasma biochemical changes in common carp (Cyprinus carpio) during cadmium exposure. Aquaculture Research 48(9): 4811-4821.
- Lu Y, Foo LY (2001) Antioxidant activities of polyphenols from sage (Salvia officinalis). Food chemistry 75(2): 197-202.
- McCay PB (1985) Vitamin E: interactions with free radicals and Ascorbate. Annual Review of Nutrition 5(1): 323-340.
- Peng S, Chen L, Qin J, Hou J, Yu N, et al. (2009) Effects of dietary vitamin E supplementation on growth performance, lipid peroxidation and tissue fatty acid composition of black sea bream (Acanthopagrus schlegeli) fed oxidized fish oil. Aquaculture Nutrition 15(3): 329-337.
- Cinar M, Yigit A, Eraslan G (2010) Effects of vitamin C or vitamin E supplementation on cadmium induced oxidative stress and anaemia in broilers. Revue de Médecinevétérinaire 161(10): 449-445.
- Montero D, Marrero M, Izquierdo M, Robaina L, Vergar JM, et al. (1999) Effect of vitamin E and C dietary supplementation on some immune parameters of gilthead seabream (Sparus aurata) juveniles subjected to crowding stress. Aquaculture 171(3-4): 269-278.
- Mehrad B, Jafaryan H, Taati M (2012) Assessment of the effects of dietary vitamin E on growth performance and reproduction of zebrafish Danio rerio (Pisces, Cyprinidae). Journal of Oceanography and Marine Science 3(1): 1-7.
- Sau S, Paul B, Mohanta K, Mohanty S (2004) Dietary vitamin E requirement, fish performance and carcass composition of rohu (Labeo rohita) fry. Aquaculture 240(1-4): 359-368.
- Harikrishnan R, Balasundaram C, Kim MC, Kim JS, Han YJ, et al. (2010) Effect of a Mixed Herb-Enriched Diet on the Innate Immune Response and Disease Resistance of Paralichthys olivaceus against Philasterides dicentrarchi Infection. J Aquat Anim Health 22(4): 235-243.
- Direkbusarakom S (2011) Application of medicinal herbs to aquaculture in Asia. Walailak Journal of Science and Technology (WJST) 1(1): 7-14.
- Ekhtiarzadeh H, AkhondzadehBasti A, Misaghi A, Sari A, Khanjari A, et al. (2012) Growth response of Vibrio parahaemolyticus and Listeria monocytogenes in salted fish fillets as affected by Zataria multifloraboiss essential oil, nisin, and their combination. Journal of Food Safety 32(3): 263-269.
- Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433: 50-61.
- Francis G, Makkar H, Becker K (2001) Effects of Quillaja saponins on growth, metabolism, egg production and muscle cholesterol in individually reared Nile tilapia (Oreochromis niloticus). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 129(2): 105-114.
- Goda A (2008) Effect of dietary Ginseng herb (Ginsana® G115) supplementation on growth, feed utilization, and hematological indices of Nile Tilapia, Oreochromis niloticus (L), fingerlings. Journal of the World Aquaculture Society 39(2): 205-214.
- Immanuel G, Uma R, Iyapparaj P, Citarasu T, Punitha Peter S, et al. (2009) Dietary medicinal plant extracts improve growth, immune activity and survival of tilapia Oreochromis mossambicus. J Fish Biol 74(7): 1462- 1475.
- Zaki M, Labib E, Nour A, Tonsy H, Mahmoud S (2012) Effect some medicinal plants diets on mono sex Nile tilapia (Oreochromis niloticus), growth performance, feed utilization and physiological parameters. APCBEE Procedia 4: 220-227.
- Yanishlieva NV, Marinova E, Pokorný J (2006) Natural antioxidants from herbs and spices. European Journal of Lipid Science and Technology 108(9): 776-793.
- Alok S, Jain SK, Verma A, Kumar M, Mahor A, et al. (2014) Herbal antioxidant in clinical practice: A review. Asian Pacific Journal of Tropical Biomedicine 4(1): 78-84.
- Niwano Y, Saito K, Yoshizaki F, Kohno M, Ozawa T (2010) Extensive screening for herbal extracts with potent antioxidant properties. J Clin Biochem Nutr 48(1): 78-84.
- Antache A, Cristea V, Grecu I, Creţu M (2014) The synergistic influence of Thymus vulgaris and vitamin E on growth performance and oxidative stress at Oreochromis niloticus species. Seria Zootehnie 62: 85-90.
- Mekkawy IA, Mahmoud UM, Wassif ET, Naguib M (2011) Effects of cadmium on some haematological and biochemical characteristics of Oreochromis niloticus (Linnaeus, 1758) dietary supplemented with tomato paste and vitamin E. Fish Physiology and Biochemistry 37(1): 71-84.
- Soleimany V, Banaee M, Mohiseni M, Nematdoost HB, Mousavi Dehmourdi L (2016) Evaluation of pre-clinical safety and toxicology of Althaea officinalis extracts as naturopathic medicine for common carp (Cyprinus carpio). Iranian Journal of Fisheries Sciences 15(2): 613-629.
- Sönmez AY, Bilen S, Alak G, Hisar O, Yanık T, et al. (2015) Growth performance and antioxidant enzyme activities in rainbow trout (Oncorhynchus mykiss) juveniles fed diets supplemented with sage, mint and thyme oils. Fish Physiology and Biochemistry 41(1): 165-175.
- Babaheydari SB, Dorafshan S, Heyrati FP, Soofiani NM (2014) Effect of wood betony (Stachys lavandulifolia Vahl) extract on some serum biochemical changes and acute stress response in juvenile common carp (Cyprinus carpio). Iranian Journal of Aquatic Animal Health 1(1): 17-26.
- Antache A, Cristea V, Iulia GR, Placinta S, Mocanu M (2013) The Influence of Rosemary, Sea Buckthorn and Ginger on Oxidative Stress at Oreochromis niloticus Reared in a Recirculating Aquaculture System. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Animal Science and Biotechnologies 70(1): 110-116.
- Yılmaz S, Ergün S, Çelik EŞ (2012) Effects of herbal supplements on growth performance of sea bass (Dicentrarchus labrax): Change in body composition and some blood parameters. J BioSci Biotech 1(3): 217-222.
- Metwally M (2009) Effect of garlic (Allium sativum) on some heavy metal (copper and zinc) induced alteration in serum lipid profile of Oreochromis niloticus. World Journal of Fish and Marine Sciences 1(1): 1-6.
- Blount JD, Metcalfe NB, Birkhead TR, Surai PF (2003) Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300(5616): 125-127.
- Abdel Tawwab M, Sharafeldin KM, Mosaad MN, Ismaiel NE (2015) Coffee bean in common carp, Cyprinuscarpio L. diets: Effect on growth performance, biochemical status, and resistance to waterborne zinc toxicity. Aquaculture 448(1): 207-213.
- Dorojan OGV, Cristea V, Creţu M, Dediu L, Docan AI, et al. (2015) The effect of thyme (Thymus vulgaris) and vitamin E on the Acipenser stellatus juvenile welfare, reared in a recirculating aquaculture. AACL Bioflux 8(2): 150-158.
- Torrissen O, Christiansen R (1995) Requirements for carotenoids in fish diets. Journal of Applied Ichthyology 11(3‐4): 225-230.
- Chainapong T, Traichaiyaporn S (2013) Enhancement of carotenoid production in Spirulina platensis and fed on Clarias macrocephalus for reproductive performance. International Journal of Agricultural Technology 9(1): 49-59.
© 2017 Mohammad Mohiseni. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






