- Submissions

Full Text
Experimental Techniques in Urology & Nephrology
Application of 3D Modeling for Preoperative Planning and Intra Operative Navigation during Procedures on the Organs of Retroperitoneal Space
Vasilii N Dubrovin1, Alexander V Egoshin1, Dmitry M Batukhtin2, Ruslan V Eruslanov2, Daniil S Chernyshov2, Alexey A Rozhentsov2, Yakov A Furman2
1State-financed Health Institution of the Republic of Mari El “Republican clinical hospital", Russia
2Volga State University of Technology, Yoshkar-Ola, Russia
*Corresponding author: Alexey A Rozhentsov, Volga State University of Technology, Yoshkar-Ola, Russia
Submission: December 13, 2017; Published: January 11, 2018
ISSN: 2578-0395 Volume1 Issue2
Abstract
Introduction: We present the original computer product "Volga-M” for the forming 3D model of kidney to conduct preoperative planning surgery and the initial clinical studies of using intra operative navigation during laparoscopic partial nephrectomy.
Materials and methods: Virtual model was formed using the software, based on the mathematical models of the structural elements of the body we accepted a law of probability distribution of the brightness values. When using augmented reality technology, we combined the 3D model image with the video of kidney tumor. Laparoscopic partial nephrectomy performed in 9 patients, 4 (44.4%) men and 5 (55.6%) women middle age 45.9 (38-54) years, with clear cell renal cell carcinoma size 26.2 (15-40) mm.
Results: All procedures were successful, segmental vascular clip was possible in 7 (53.8%) cases. Mean warm ischemia was 17.3 (12-25) min. Mean surgery operation time was 101.7 (80-155) minutes. Blood loss was an average of 222.2 (100-400) ml.
Conclusion: Preliminary results of our clinical studies have shown the significance of 3D modeling to improve visualization of the affected organ during surgery for the surgeon and for understanding of the nature of the pathological process of the patient
Keywords: Laparoscopic urology; Virtual modeling; Augmented reality; Intraoperative navigation
Abbreviations: LPN: Laparoscopic Partial Nephrectomy; CT: Computer Tomography
Introduction
Modern development video-endoscopic technologies has undoubted benefits for the patient but has features that hinders the surgeon's work. The most significant of these occur when we are working with 2D and pseudo-3D images of the surgical operation zone on the monitor, with the inability of the surgeon to use the tactile sensitivity, leading to difficulties of orientation in operating space [1]. The development of computer technologies and methods of radiation diagnosis will allow create a 3D model for the surgery based on the results of a standart cross-sectional computer tomography (CT) scan. The analysis of 3D models of body region of interest that is viewed on a computer screen or is printed on a 3D printer helps the surgeon to assess features of the upcoming surgery and the patient can understand the disease and its treatment [2].
The great advantage of virtual simulation is an opportunity to extract the outer contour of the body of interest as well as to study its internal structure, particularly the blood supply, which is especially important during organ-preserving surgery for kidney tumor. During the partial nephrectomy processing the preoperative planning can reduce kidney warm ischemia time which improves the results of treatment [3]. Especially promising is the use of virtual models directly in the video-endoscopic surgery for intraoperative navigation, which gives the surgeon additional information about the individual patient's anatomy [4]. It is applied a different options based on augmented reality technology. They include CT examination, recording the results in the format of DICOM, the forming of a virtual 3D model corresponding to the individual anatomy of the patient and the matching to 3D models with real video image of the patient. Image synthesis is performed using a virtual model of the projection on sub-screen monitor, directly on the patient body or on a video monitor in laparoscopic surgery [5]. In this paper, we present an original hardware-software product "Volga-M” (cert.N 20156604626, 2015), that allows to form a 3D model from the data obtained at CT, to conduct preoperative planning surgery using a virtual model on the screen and the initial clinical studies of using intraoperative navigation during laparoscopic partial nephrectomy [6].
Materials and Methods
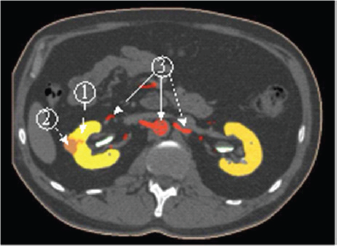
To increase the visibility of CT data we suggest the using of 3D model obtained by the results of the segmentation of tomographic images. The segmentation is based on the difference between the statistical properties of the brightness of individual segments of body tissue. As the mathematical models of the structural elements of the body we accepted a law of probability distribution of the brightness values. We confirmed the normal nature of these distributions experimentally. Mathematical models of organs were specified by the results of the calculation of their average brightness and variance. The labeling of a single point of CT image to one of the elements of the body are performed by the maximum likelihood accessories this point of each of the elements (Figure 1).
Figure 1: The segmentation of the surgical zone: 1-kidney, 2-tumor, 3-arteries.
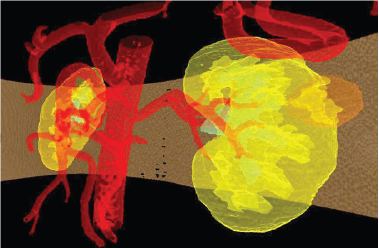
The 3D image model of the kidney and its structural elements are formed from a package of tomographic slices on which is made the segmentation and are preserved a slice ordering (Figure 2).
Figure 2: The synthesized model of kidney and tumor in a transparent mode.
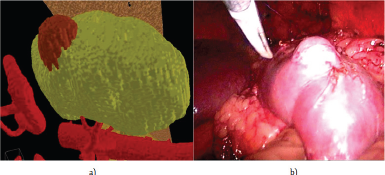
Virtual model of kidney and surgical areas of interest allows the surgeon to study in details the features of the disease, to obtain three-dimensional model body. This image demonstrated to the patient and his family to better understanding the nature of the disease. The image of virtual model of kidney demonstrated in operation room on the screen nearby the video image obtained with laparoscopic camera in the corresponding projection to facilitate the orientation in the retroperitoneal space (Figure 3).
Figure 3: 3D model image during laparoscopic surgery: a) the 3D model of kidney and tumor, b) surgical video.
The model was used for preoperative planning partial nephrectomy. On virtual model of the kidney was removed a tumor with minimal damage to the normal renal parenchyma. Preoperative planning of surgical intervention has allowed assess the possible risks during the upcoming operation associated with damage to major blood vessels of the kidney cavity. We used the original method of combining images obtained during video- endoscopic operations from the video sensor, with images of a virtual 3D model of the body. The position of the laparoscope in the retroperitoneal space is determined using 3D digitizer, combined with a video camera(Figure4).
Figure 4: 3D-digitazer Microscribe G2 with laparoscope.

Figure 5: Combining images of 3D models with video of kidney.
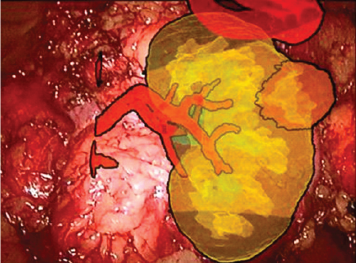
Real laparoscopic camera calibrated for combination image that allowed synchronously change the parameters of the virtual camera, ensuring receipt of the combined image of the virtual and the real object. Imaging takes place in real time and provides a continuous video stream. When using augmented reality technology combined image of the 3D model with the video kidney tumor (Figure 5).
Clinical Experience
Nine patients underwent transperitoneal laparoscopic partial nephrectomy (LPN), among whom men were 4 (44.4%), women-5 (55.6%), and middle age 45.9 (38-54) years with clear cell renal cell carcinoma size 26.2 (15-40) mm. To conduct the study we obtained the approval of the local ethics committee of the Republican Clinical Hospital of Mari El Republic, the voluntary consent obtained from all patients. All patients underwent standard clinical examination, including standart cross-sectional CT (Siemens Sonotom 3000 Philips Brilliance 64), the results of which were recorded in DICOM format. Virtual model of the kidney and the area of surgical intervention were formed using the original software "Volga-M”. A 3D-model of kidney tumors has been demonstrated for patients and their families to better understand the nature of the disease, tumor localization, size and characteristics of the upcoming surgery.
We examined the demographic, intraoperative, and postoperative indicators of patients, including kidney warm ischemia time, operative time, postoperative histology data, the surgical margins and postoperative complications (Table 1). During the preoperative surgical planning with using a 3D model the nature of the disease, the forthcoming intervention and its features have been discussed with the patient. In all cases, the patient understands the essence of the disease and the characteristics of the upcoming surgery.
Table 1: Demographic, tumor characteristics, operative and perioperative patient data, pathologic outcomes of patients undergoing laparoscopic partial nephrectomy with preoperative planning and intraoperative navigation.
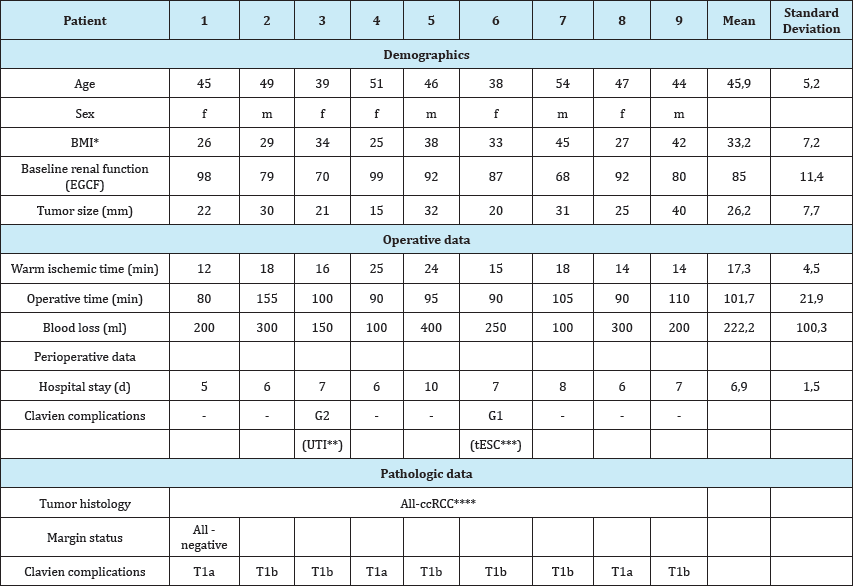
*BMI: Body Mass Index, **UTI: Urinary Tract Infection, ***tESC: Transient Elevation of Serum Creatinine; ****ccRCC: Clear Cell Renal Cell Carcinoma.
Results
All patients successfully underwent laparoscopic partial nephrectomy. During laparoscopic surgery the segmental vascular clip imposing on the renal artery was possible in 4 (45%) cases, the remaining five (55%) cases-clamp was applied to the renal artery Partial nephrectomy was performed with cold scissors. Collecting system has not been opened in all cases. Hemostatic suture fixation with the plastic clips was applied (vicril 2/0, Hem-o-Lock). Mean warm ischemia was 17.3 (12-25) minutes. Mean surgery operation time was 101.7 (80-155) minutes. Blood loss was an average of 222.2 (100-400) ml. The patients were carried out early activation the next day after procedure. No serious postoperative complications were not observed, the patient #6 had a transient elevation of serum creatinine, did not require special treatment (G1), the patient #3 had urinary tract infection, antibiotic therapy is appointed (G2). All patients diagnosed with clear cell renal cell carcinoma, there were no cases of positive surgical margins histologically. The average duration of treatment was 6.9 (5-10) days. Preoperative patient demographics, tumor characteristics, operative data, perioperative data, pathologic outcomes for each patient are described in the Table 1.
Discussion
The 3D model of the operative zone obtained before surgery has allowed preoperative planning before LPN in which clearly was determined a tumor location, its connection with the arteries of the renal parenchyma, renal cavity systems. Possessing the virtual removal of the tumor could be observed the possible damage to the internal structures of the kidney, be determined their location and methods to predict the elimination of possible complications.
Application of the method of combining the computer image obtained by CT and images on the screen when the video-endoscopic surgery allowed the surgeon to better represent the individual anatomy of the operated organ and it's angio-architectonic, kidney tumor location, its connection with the blood vessels, allowing for a partial nephrectomy radical within the healthy tissue. The ability to determine precisely the segmental artery provides a significant advantage in terms of preserving renal function after the surgery. The warm ischemia undergoes not all kidney's parenchyma, but only the segment affected tumor process and this segment be deleted. In addition, following the principles of nephron-sparing surgery, it is important not to remove a significant portion of unaffected renal parenchyma to save a total of renal function postoperatively.
In our study, all 9 patients successfully underwent laparoscopic partial nephrectomy regarding clear cell RCC. After separating a kidney vessels vascular clamping of segmental arteries that was possible in 45.0% cases, the mean warm ischemia time was 17.3 (12-25) minutes, blood loss was an average of 222.2 (100-400) ml, we believe that this contributed to the improved visualization during surgery due to augmented reality. Augmented reality is increasingly used in various areas of medical practice, including in urology and is a combination of modern computer technology and medical imaging [7-9]. Creating 3D models of an organ or area of surgery based on CT studies recorded in the format of DICOM allows combine the different phases of contrast studies, including vascular, parenchymal and excretory. This virtual model demonstrated on the screen or printed on a 3D printer, more approximates to real organ and gives the surgeon additional information and it is useful for the understanding of patient illness [10]. The study of renal arterial tree using 3D modeling perspective for model with partial nephrectomy for maximal nephron sparing surgery [11]. Modern development video-endoscopic technology in minimally invasive surgery has really advantages for the patient. However, the use of endoscopic technologies creates additional difficulties associated with new unusual visualization, because the surgeon watching the actions on the screen with a 2D image. During laparoscopic surgery, the surgeon does not feel "deep" wounds, field of view is limited to the field of view of the camera, and there is no tactile sensitivity. In this situation, any additional information about the anatomy of the individual areas of surgery is extremely helpful.
Using a virtual 3D model, augmented reality helps to emphasize the contours of the body, the boundaries of the pathological process, allows us to see the internal structures in the "transparent” mode (Figure 5), which is especially valuable in partial nephrectomy [12]. Application of augmented reality technology in retroperitoneal surgery is difficult because there is a constant shift of the respiratory organs during the tour, with surgical procedures, there are no permanent fixed reference points, surgical field is located in the adipose tissue. The most difficult problem is the matching the 3D models and images of real organ on the monitor during endoscopic operations in real time. Image synthesis and virtual model of the real body is carried out using a see-through optical display, for projecting the virtual model [13]. Some authors use the method when the projector is positioned over the patient and the virtual model is projected onto the patient's skin [14]. The monitor is the main source of information for the surgeon during the video-endoscopic procedure, so many authors have used it for visualization 3D models [15]. The image pattern may be superimposed on a video image transmitted to the screen or the sub-screen monitor [16].
In 2009, Su et al. successfully applied the technology of augmented reality with the robot-assisted partial nephrectomy, using the imposition of 3D reconstructed CT on the video in real time [17]. Program platforms allowing create virtual models of organs or areas of surgical interest on the basis of CT study, which are not tied directly to the CT machine such as Tile Pro, OsiriX successfully used in laparoscopic and robot-assisted operations, including the partial nephrectomy [18,19]. In our study, we used the original method for forming a virtual model based on preoperative CT studies. Preliminary we used modeling to determine the optimal access point to the area of interest in the surgical minimally invasive surgery [20]. Further experimental work carried out to improve the quality of the image, the automatic segmentation of the body to adapt the virtual model to be printed on 3D printer. Currently, research continues towards conjugation real and virtual video image in real time.
Conclusion
Preliminary results of our clinical studies have shown the significance and success of 3D modeling to qualitative visualization of the affected organ during surgery for the surgeon and for the understanding of the nature of the pathological process of the patient. Hardware-software complex "Volga-M” provides additional information about the location of the kidney tumor directly during surgery by combining the virtual model and the video image. Further improvement of our method is promising improvement of the results of operations on organs of the retroperitoneal space. We plan to continue observation and increase the number of patients to evaluate the long term result of the patient's cancer free status.
Acknowledgments
The work was carried out with the financial support of the Ministry of Education and Science of the Russian Federation in the framework of the implementation of the Federal target program "Research and development in priority areas for the development of Russia’s scientific and technical complex for 2014-2020 ", the project RFMEFI57717X0254 "System of intraoperational navigation with support of the technology of augmented realness on the basis of virtual 3D models of organs ", obtained by the results of CT diagnostics, for small invasive operations.
References
- Ukimura O, Gill IS (2009) Image-fusion, augmented reality, and predictive surgical navigation. The Urologic clinics of North America 36(2): 115-123.
- Marescaux J, Diana M, Soler L (2013) Augmented Reality and Minimally Invasive Surgery. Gastroenterology and Hepatology Research 2(5): 555560.
- Rassweiler J, Rassweiler M, Müller M, Kenngott H, Meinzer HP, et al. (2014) Surgical navigation in urology. European perspective. Current Opinion in Urology 24(1): 81-97.
- Teber D, Guven S, Simpfendörfer T, Baumhauer M, Güven EO, et al. (2009) Augmented reality: a new tool to improve surgical accuracy during laparoscopic partial nephrectomy? Preliminary in-vitro and invivo results. Eur Urol 56(2): 332-338.
- Nakamoto M, Ukimura O, Faber K, Gill IS (2012) Current progress on augmented reality visualization in endoscopic surgery. Current Opinion in Urology 22(2): 121-126.
- Dubrovin VN, Batukhtin DM, Yegoshin AV (2004) Preoperative planning and intraoperative navigation, based on 3D modeling for retroperitoneal procedures. 3D reconstruction. Techniques, analysis and new developments. New York, USA, pp. 1-38.
- Shuhaiber J (2004) Augmented reality in surgery. Archives of Surgery 139(2): 170-174.
- Nicolau S, Soler L, Mutter D, Marescaux J (2011) Augmented reality in laparoscopic surgical oncology. Surgical Oncology 20(93): 189-201.
- Soler L, Marescaux J (2008) Patient-specific surgical simulation. World Journal of Surgery 32(2): 208-212.
- Silberstein J, Maddox M, Dorsey P, Feibus A, Thomas R, et al. (2014) Physical models of renal malignancies using standsrt cross-sectional imaging and 3Dimentional printers: a pilot study. Urology 84(2): 268273.
- Drewniak T, Rzepecki M, Juszczak K, Moczulski Z, Reczynska K, et al. (2013) Methodology and evaluation of the renal arterial system. Central European Journal of Urology 66(2): 152-157.
- Hughes-HA, Mayer E, Marcus HJ, Cundy TP, Pratt PJ, et al. (2014) Augmented reality partial nephrectomy: Examining the current status and future perspectives. Urology 83(2): 266-273.
- Okamoto T, Onda S, Matsumoto M, Gocho T, Futagawa Y, et al. (2013) Utility of augmented reality system in hepatobiliary surgery. Journal of Hepato-Biliary-Pancreatic Sciences 20(2): 249-253.
- Sugimoto M, Yasuda H, Koda K, Suzuki M, Yamazaki M, et al. (2010) Image overlay navigation by markerless surface registration in gastrointestinal, hepatobiliary and pancreatic surgery. Journal of Hepato-Biliary- Pancreatic Sciences 17(5): 629-639.
- Marescaux J, Rubino F, Arenas M, Mutter D, Soler L (2004) Augmented- reality-assisted laparoscopic adrenalectomy. JAMA 292(18): 2214-2215.
- Hughes HA, Pratt P, Mayer S, Martin E, Darzi A, et al. (2014) Image guidance for all-TilePro display of 3-dimensionally reconstructed images in robotic partial nephrectomy. Urology 84(1): 237-242.
- Su L, Vagvolgyi B, Agarwal R, Reiley CE, Taylor RH, et al. (2009) Augmented reality during robot-assisted laparoscopic partial nephrectomy: toward real-time 3DCT to stereoscopic video registration. Urology 73(4): 896900.
- Volonte F, Buch N, Pugin F, Spaltenstein J, Schiltz B, et al. (2013) Augmented reality to the rescue of the minimally invasive surgeon. The usefulness of the interposition of stereoscopic images in the Da VinciTM robotic concole. The International Journal of Medical Robotics 9(3): 3438.
- Lasser MS, Doscher M, Keehn A, Chernyak V, Garfein E, et al. (2012) Virtual surgical planning: a novel aid to robot-assisted laparoscopic partial nephrectomy. Journal of Endourology 26(10): 1372-1379.
- Dubrovin V, Bashirov V, Furman Y, Rozhentsov AA, Yeruslanov RV, et al. (2013) Choice of surgical access for retroperitoneoscopic ureterolithotomy according to the results of 3D reconstruction of operational zone agreed with the patient: initial experience. Central European Journal of Urology 66(4): 447-452.
© 2018 Vasilii N Dubrovin, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






