- Submissions

Full Text
Experimental Techniques in Urology & Nephrology
Loin Pain and Haematuria Syndrome (LPHS) Linked to Symptomatic Nephroptosis (SN) and Revealing Pedicle Stretch Causing Neuro-Ischaemia Using the New IVU 7 Sign
Salma A Ghanem1, Khalid A Ghanem2, Nisha Pindoria3 and Ahmed N Ghanem4*
1Royal London Hospital, London
2Mansoura University Hospital, Egypt
3North Middlesex University Hospital, London
4Retired Consultant Urologist, Egypt
*Corresponding author: Ahmed N Ghanem, MD, FRCS, Retired Consultant Urologist, No1 President Mubarak Street, Mansoura 35511, Egypt
Submission: July 27, 2017; Published: October 02, 2017
ISSN: 2578-0395 Volume1 Issue1
Abstract
This article is based on a prospective 10 years observational study on loin pain haematuria syndrome (LPHS) complicating symptomatic nephroptosis (SN) and the results of renal sympathetic denervation and nephropexy (RSD&N) surgery [1,2]. The objective here is to demonstrate that renal pedicle stretch causes neuroischaemia as evidenced by the new IVU 7 sign.
Episodic loin pain (LP) or LPHS that has no detectable pathology is frustrating to both patient and urologist. I inherited 30 cases from my predecessor at King Khaled Hospital, Najran, Saudi Arabia. Repeated investigations and imaging were all normal. A patient showed me a maneuver to bring her own right kidney down to right iliac fossa and keeping it there for examination (Loin grip sign). Ever since I diagnosed 1.7new cases/m and found the underlying cause of SN and LPHS.
The main management problem of loin pain was the lack of demonstrable pathology on repeated imaging, when supine. The underlying SN though well known [3] was disparaged [4] and LPHS though well documented, its existence may be doubted [5] and both are extremely problematic to manage [5-8].
Demonstrable renal pathology of loin pain and haematuria was invariably lacking on all supine imaging of the received protocol [5- 8]. Urinary tract infections (UTI) may affect a few patients during the occasional episodes but UTI, stones and organic causes play no role in the pathogenesis of LPHS. Many complex ramifying management problems of SN and LPHS are well known, but have no solutions. Some of the problems were communicated [9,10] and the illusive overlooked link of SN with LPHS was pointed out recently [1,2].
The objective of this study was to answer the following questions:
a. Is chronic episodic LP/LPHS genuine?
b. Is there an organic pathology? How to reveal it?
c. Why is the Standard Imaging Protocol (SIP) negative?
Defining
a. Nephroptosis means mobile kidney.
b. Standard Imaging Protocol (SIP) as in current use.
c. Upright Imaging Protocol (UIP) in which an IVU with erect film (IVU-E) is done.
Demonstrating
a. Features and complications of SN seen on UIP not on SIP
b. Patho-etiology link of SN with LPHS.
Discussing
a. Reasons for disparaging SN, ignoring it by Textbooks and overlooking it in routine urological practice
b. USA Authorities ignores both SN & LPHS, UK Authorities ignores SN and what the leaders choose to ignore, others do not see.
Nephroptosis is differentially diagnosed from ectopic Kidney by blood supply and mobility. In SN the kidney drops >1.5v (>5cm) on erect IVU film causing pain with/out haematuria. In LPHS no detectable anomalies is seen on SIP. Every pair of IVU films, shown here, shows left Supine (S) and right Erect (E) for comparison.
Measuring renal drop, laterality, pelvi-ureteric junction (PUJ) kink and pelvicalyceal dilatation (PC) as evidence of obstruction on IVU E, have established renal pedicle stretch and rotation as evidence of renal neuro-ischaemia. Although SN is known, it was disparaged >60 years ago and omitted from all textbooks. SIP is constantly normal- at supine posture. UIP (IVU-E) is rarely requested, hence chance diagnosis of SN is unlikely and diagnosis is easily missed. Many of SN features and some complications were documented >70 years ago. The link of SN with LPHS is a new discovery explaining its real patho-etiology [1,2].
Patients and Methods
This is based on a prospective observational 10 years study of 236 patients presenting with episodic LP/LPHS. All had NO detectable anomalies or known organic causes of LP/LPHS on SIP. Of presented cases, 190 demonstrated anomalies on UIP of IVU-E and retrograde pyelography (RGP). All 190 SN patients had renal drop >1.5 and up to 5 vertebrae (>5 to 17.5cm) on IVU-E film. Most patients also demonstrated other features and complications on UIP, while SIP is negative as it is routinely done at Supine (S) posture. KUB, US & IVU were normal when S. Ancillary imaging of CT, MRI & Isotope Renography (IR) were also normal at S posture. UIP is the same as SIP after adding erect (E) IVU film. IVU-S was repeated with erect film at 15min (IVU-E). Isotope renography (IR) was done at S and E (sitting) postures. Retrograde pyelography (RGP) with Cystoscopy was done on patients who consented.
In order to affirm LP/LPHS is genuine we developed the following new methods
a. Loin Grip Sign gripping the loin while the patient in standing position holds the kidney at ptosis position after lying supine, for examination at right iliac fossa.
b. One step jump test asking the patient to jump from one step to the floor causes agony which affirms that pain is real and genuine.
To affirm pedicle stretch causes ischaemia
i. The IVU 7 sign, mapping the renal pedicle on IVU-S and IVU-E demonstrate its degree of stretch
ii. Rubber tube stretch hypothesis shows that stretch narrows the lumen of a tube and induces renal ischaemia.
The kidney was explored by open surgery, placing the kidney at ptosed position caused stretch of renal pedicle causing ischaemic cyanosis of the kidney. This confirmed the IVU 7 sign and tube stretch hypothesis; representing the renal pedicle stretch ischemia and neuropathy. The kidney was explored through loin incision. The renal artery was skeletonised cutting and diathermy all surrounding nerves. Nephropexy was done by the 3 point technique
Results
Figure 1: Shows frequency distribution of patients’ age.
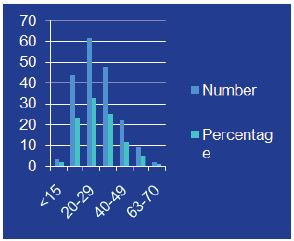
Figure 2: Shows frequency distribution of renal drop in vertebrae.
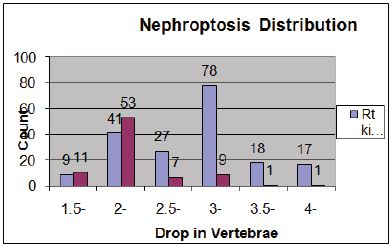
Figure 3: Compares IVU-S with IVU-E shows right nephroptosis of 2 vertebrae.
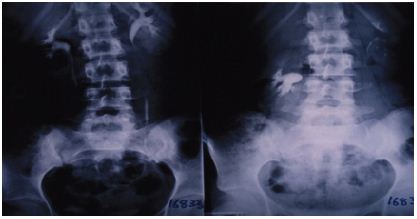
Figure 4: Compares IVU-S with IVU-E shows bilateral nephroptosis of 3 and 2 vertabrae.

TOf 190 patients with SN on IVU-E, 182 were females and 8 males. Of 46 excluded cases, 26 had SN <1.5v, 11 undetermined (allergy) and 9 had urinary stones. A drop of >3 veretebrae (10.5cm) affected 124 (65%). Mean age was 28.7 (12-70), hospital follow up 6.6 (3- 16) and symptom duration 15.7 (3-46) y. Age distribution is shown in Figure 1 and ptosis distribution is shown in Figure 2. Ptosis affected right kidney alone (Figure 3) in 107/190 (56.3%) and was bilateral (Figure 4) in 82 (43.2%) patients.
The patients were segregated by presentation into two groups. The Emergency Room (ER) group of patients was mostly young, thin and single females. Their mean body weight 47.7kg, height 155cm and body mass index (BMI) 19.8kg/m2. Severe LP/LPHS episodes required repeated hospital admissions of 97 (51%). Haematuria episodes affected 36 (18.9%) patients. LP affected all right sides of ptosis but may affect the left side as part of bilateral involvement. False diagnosis during an episode of the acute abdomen caused unnecessary surgeries including appendecectomy in 28, intervertebral disc removal in 3 and cholecystectomy in 4 patients. False label included depression in 18, opiate dependency in 13 and malingering in 3 patients.
The out-patient department (OPD) group regularly attended clinics with episodes of LP and the multiple associated splanchnic symptoms (MASS). They were married multi-parous women with a mean body weight of 66kg, height 155cm and BMI 28 kg/m2. They regularly attended many clinics of medical, gastro-intestinal tract (GIT), Nephrology, UTI, Backache and Pain clinic.
MASS indicated sympathetic over-activity and included:
GIT symptoms of acid peptic disease (APD) and irritable bowel syndrome (IBS) affected 94 (49%) patients.
b. Backache affected 120 (63%) of whom 24 (12.6%) patients had sciatica-like pain (normal CT spine).
c. Cystitis without UTI affected 96 (51%) patients, of whom 12 had bladder biopsy and showed interstitial cystitis.
Presentation
i. Recurrent attacks of severe LP or LPHS to OPD or ER
ii. ER Group had the differential diagnosis of right acute abdomen.
ii. OPD Group had LP and MASS of GIT of APD / IBS, Backache and Cystitis. All specialist investigations were normal.
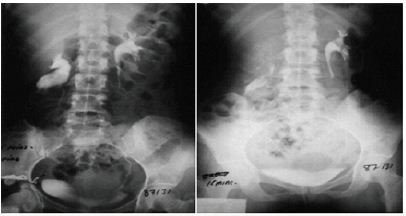
Kink at PUJ was demonstrable on IVU-E of 135/190 (71.1%), 116 (61.1%) right and 19 (10%) bilateral. PUJ kink with renal pelvis dilatation on IVU-E affected 46 (24.2%) patients, 37 right and 9 bilateral kidneys. Renal pelvis dilatation became evident on follow up later on IVU-S of 18 (9.5%) and classical PJU obstruction developed in 7 cases (Figure 5).
Figure 5: Shows IVU with nephroptosis complicated with PUJ obstruction.
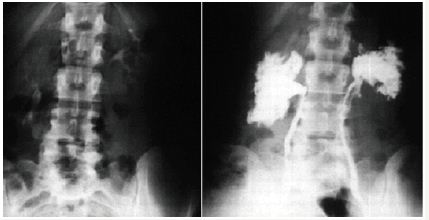
Ischaemia due to pedicle stretch and renal twist torsion rotation around pedicle demonstrated in 124 (65.3%) patients. The renal ischaemia is evidenced by the IVU 7 sign and tube stretch hypothesis (Figure 6 & 7). Ischaemia caused erosion of medullary papillae best shown on RGP (Figures 8 & 9).
Figure 6: Shows renal pedicle mapped on a supine IVU film (Horizontal) and erect film (Vertical) where the renal pedicle is stretched to 3 times its normal length.
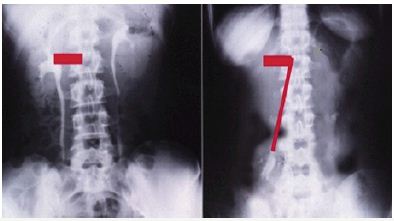
Figure 7: Compares the rubber tube stretch with IVU 7 sign which demonstrate that stretch of a tube or artery causes stenosis of the lumen.

Figure 8: Shows RGP with multiple pyelocalyctaisis with erosion of medullary papillae and contrast leakage into veins (papillary venous fistula).
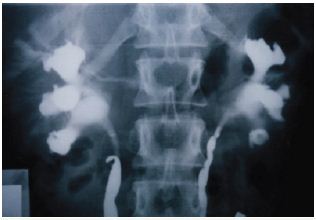
Figure 9: Compares IVU with RGP of the same patient, IVU looks normal while RGP reveals extensive bilateral renal damage (eroded papillae of pyelocayctaisis).
The 99mTc-DTPA IR at supine and seated posture showing blood flow, GFR and washout curves. Heterogeneous causes of LP and LPHS in SN demonstrable on IR but are posture dependant. Split kidney function was shown on changing posture from S to E (sitting). The pathology shown on IR may show ischaemia (Blood Flow & GFR) and or obstruction (Drainage) (Figure 10).
Figure 10: Shows isotope renography at supine and sitting postures with split renal function.
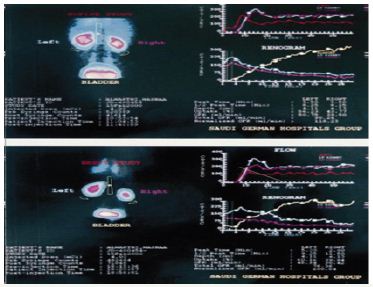
Figure 11: Shows agony on a patient’s face who suffered from LPHS after jumping from one step to the floor

Open surgical treatment was used in 28 patients suffering from severe LPHS; 10 had simple nephropexy and 18 had RSD&N. Four of those treated with simple nephropexy had recurrence of LPHS while those who had RSD&N were all cured of LPHS. Nephropexy alone is inadequate treatment. The evidence that repeated stretch of renal pedicle causes ischaemia and focal infarctions of the kidney is shown in Figure 11 as a result of the severe vascular stenosis affecting renal Pedicle (Figure 12). The new physical signs that demonstrate LP and LPHS are genuine are shown in (Figures 13 & 14).
Figure 12: Shows the ioin grip sign applied when the patient is standing then keep it as she lies supine (Top) to allow for kidney examination at the right iliac fosse (bottom).
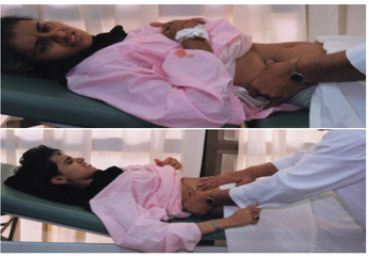
Figure 13: Shows an explored right kidney of LPHS patient which is cyanotic with focal infarction.
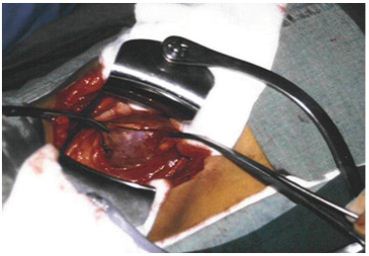
Figure 14: Shows an explored right renal pedicle after denervation of LPHS patient shown in Figure 13 with severely stenosed artery and vein (effect of chronic repeated pedicle stretch). The Nelaton tube is approximately the size of a normal renal artery.

Discussion
The IVU 7 sign and tube stretch shows that stretch to twice the normal length reduces artery diameter to ½, the cross section area to ¼ and flow to 1/16(6.25%). Artery twist/torsion induces further ischaemia. The renal pedicle contains renal artery, vein and sympathetic nerves. Thus SN initially causes functional stretch neuropathy and ischaemia explaining pain and haematuria of LPHS. It later causes ischameic damage to renal papillae.
This causes sympathetic neuropathy and nephropathy inducing ischaemia of SN which is initially intermittent and functional causing LP/LPHS. LPHS takes 3-4 years to complicate SN. Ischaemia organic permanent calyctaisis becomes evident on RGP: It starts at upper right calyx, common site of haematuria, spreads to other calyx of the same right kidney and affects the medullary papillae long before cortex. Auto-nephropexy detected in 12 (6.3%) patients and auto-nephrectomy (Renal atrophy) in 4% of patients. Focal or segmental infarctions may occur. Retrograde pyelography demonstrates the organic pyelocalyctaisis (Figure 8 & 9) as the patho-etiology of painful hematuria, caused initially by intermittent renal ischemia of pedicle ptotic stretch or torsion [11,12]. Also PUJ kink obstruction may occur with nephroptosis which is initially functional intermittent and later cause organic PC dilatation.
The reported patho-etiology of SN pain is heterogeneous with obstruction, ischemia and neuropathy components that have reversible and organic stages. Although, these causes, and rarely UTI, may contribute to the establishment of pyelocalyctaisis, [13- 15] the lesion seems primarily ischemic [10-12]. The link of SN with LPHS was illusive and overlooked, particularly when the ischemic complications of auto-nephropexy, auto-nephrectomy and sympathetic nephroplegia involving the normal contra-lateral kidney occurred insidiously over many years [1]. Auto-nephropexy, made the link of SN with LPHS most elusive by erasing the evidence on renal mobility, but demonstrated that simple nephropexy [14- 16] or spontaneous nephropexy alone may neither cure the loin pain of SN nor abort its complication into LPHS.
The demonstrable pathology on upright IVU, [2,3,9-16] arteriography [11,12] and IR [2,13] are well documented on SN that was discarded long ago [4]. Upright imaging is currently undone and has not been reported previously in LPHS. Retrograde pyelography findings (Figure 8 & 9) have not previously been documented in either condition. The use of IVU started early in the 20th century while clinical evidence on the genuineness of SN pain dated back to the 15th century [15-17]. Loin pain hematuria syndrome was reported in 19675 while Dietl’s crisis is known for centuries. Organic reno-vascular complications demonstrated on conventional arteriography of SN [11,12] and LPHS [5-8] are of advanced cases. The demonstrable link of SN with LPHS, other ischemic complications of infarction and atrophy “auto-nephrectomy” and the most elusive “autonephropexy” and “sympathetic nephroplegia” are reported here. Hahn [18] reported nephropexy for SN in 1881 and Blacklock [19] reported renal sympathetic denervation in 1989. The IVU 7 sign (Figure 6 & 7) and deformity of renal calyxes seen on erect IVU film demonstrate the renal pedicle stretch and torsion, respectively, which are important in explaining the neuro-ischhamic pathology of LPHS.
Conclusion
LP and LPHS are genuine symptoms of SN. SN is overlooked on SIP but demonstrable on UIP. The features and complications of SN cause genuine incapacitating episodic LP/LPHS. Pain of SN has heterogeneous causes of intermittent (functional) or permanent (organic) and of obstructive and neuro-ischaemic origin. PUJ kink causes Obstruction. Ischaemic stretch and/or twist torsion of renal pedicle causes neuro-ischaemic nephropathy via sympathetic over-stimulation and neuropathy. SN may complicate into LPHS causing ischaemic papillary necrosis calyctaisis and veno-calyceal shunt which is evident on RGP. The neuro-ishaemia due to pedicle stretch/rotation explains the LPHS.
The IVU-E and RGP demonstrate these features of SN while all ancillary imaging remain normal being supine. The IVU 7 sign and calyx deformity are used for detecting stretch neuro-ischaemia and obstructive pathology, respectively. The condition goes through two stages; a reversible stage which is curable and an irreversible stage which is incurable. IVU-E film and RGP are indicated in all LPHS cases. Renal sympathetic denervation and nephropexy (RSD&N) offer the best chance of curing SN and LPHS. In view of the new evidence presented here, the authorities should reconsider SN with link to LPHS and bring it back to current textbooks.
References
- Ghanem AN (2002) Feature and complications of symptomatic nephroptosis causing the loin pain haematuria syndrome: Preleminary report. Saudi Med J 23 (2): 197-205.
- Ghanem SA, Ghanem AN (2016) Prospective Observational Study on Loin Pain Hematuria Syndrome Complicating Symptomatic Nephroptosis and the Results of Renal Sympathetic denervation and Nephropexy Surgery. J J Nephro Urol 3(1): 025.
- Burford CE (1946) Nephroptosis with coexisting renal lesions. J Urol 55: 220-224.
- Hoenig DM, Hemal AK, Shalhav AL, Clayman RV (1999) Nephroptosis: A disparaged condition revisited. Urology 54(4): 590-596.
- Little PJ, Sloper JS, de Wardner HE (1967) A syndrome of loin pain haematuria associated with disease of the peripheral renal arteries. Q J Med 36(142): 253-259.
- Armstrong T, McLean AD, Hayes M, Morgan BT, Tullock DN (2000) Early experience of intrauterine capsaicin infusion in loin pain haematuria syndrome. BJU Int 85(3): 233-237.
- Editorial (1992) Loin pain haematuria syndrome. Lancet 340 (ii): 701- 702.
- Andrews BT, Jones NF, Browse NL (1997) The use of surgical sympathectomy in the treatment of chronic renal pain. Br J Urol 80(1): 6-10..
- Ghanem AN (2000) Disparaged Nephroptosis. Urology 56(1): 183-184.
- Ghanem AN (2000) Early experience of intrauterine capsaicin infusion in loin pain haematuria syndrome. BJU Int 86: 911-914.
- Kaufman JJ, Hanafee W, Maxwell MH (1964) Upright renal arteriography in the study of renal hypertension. JAMA 187: 977-980.
- Stoll HG (1970) Indications of Nephropexy with special reference to the renovascular aspects of ptosis. Der Urologe A 9Jg. Heft 3: 114-117.
- O‘Reilly PH, Pollard AJ (1988) Nephroptosis: a cause of renal pain and a potential cause of inaccurate split renal function determination. Br J Urol 61(4): 284-288.
- McWinnie DL, Hamilton DNH (1984) The rise and fall of the floating kidney. Br Med J 288(6420): 845-847.
- Braasch WF, Greene LF, Goyanna R (1948) Renal ptosis and its treatment. JAMA 138(6): 399-403.
- Deming CL (1930) Nephroptosis: causes, relation to other vescera and correction by a new operation. JAMA 95(4): 251-257.
- Mathe CP, Sanchez DLPL (1957) Orthostatic renal hypertension resulting from torsion and ptosis of kidney. J Int Coll Surg 27(1): 36-45.
- Hahn E (1881) Die operative Behandlung der beweglichen Niere Durch fixation. Zintralbl Chir 29: 449-452.
- Blacklock ARE (1989) Renal denervation with releasing renal capsule incision in the loin pain haematuria syndrome. Br J Urol 64(2): 203-204.
© 2017 Salma A Ghanem, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






