- Submissions

Full Text
Experiments in Rhinology & Otolaryngology
Microbial Flora in Chronic Rhinosinusitis with and without Nasalpolyps
José Gameirodos Santos1*, Rosário Figueirinhas1, João Carvalho Almeida1, Cláudia Santos2, Amílcar Falcão3, Corália Vicente4, João Paço5 and Cecília Almeida eSousa1
1Department of Otolaryngology-Head and Neck Surgery, CHP Hospital Santo António, Portugal
2Department of Microbiology, CHP Hospital Santo António, Portugal
3Faculty of Pharmacy, University of Coimbra, Portugal
4Abel Salazar Biomedical Sciences Institute, Portugal
5CUF Infante Santo Hospital, Portugal
*Corresponding author: José Gameirodos Santos, Department of Otolaryngology-Head and Neck Surgery, CHP-Hospital Santo António, Serviçode ORL–CHPP porto Largo Prof Abel Salazar 4099-001-Porto, Portugal
Submission: September 09, 2017;Published: October 27, 2017

ISSN: 2637-7780Volume1 Issue1
Abstract
Introduction: The most common microbial agents in the etiology of chronic rhinosinusitis are defined in the literature as Staphylococcus aureus, Staphylococcus coagulase-negative and Streptococcus spp. In healthy individuals these same microorganisms are also the most frequent (mainly Staphylococcus coagulase negative) ascolonizing flora agents. We often encounter a poly microbial colonization of the nose and sinuses. The contribution of the different pathogens for the disease remains sun certain. The aim of this study is to compare the microbial flora found in patients with chronic rhinosinusitis with and without nasal polyps.
Methods: Prospective clinical study. 110 patients with indication for endonasal microsurgery by chronic rhinosinusitis were evaluated. Patients were divided into two groups: with nasalpolyps (70) and without nasalpolyps (40). During surgery, mucosa of the middle meat us/anterior ethmoid was collected, in the side with the highest score Lund-McKay and sent for bacteriological analysis.
Results: There was a predominance of aerobic Gram-positive bacteria, followed by aerobic Gram-negative and an aerobic bacteria. When evaluated separately the groups with and without nasalpolyps, there was a predominance, in both, of Gram-positive bacteria. However, in the group with nasalpolyps, these cond higher prevalence was of aerobic Gram-negative bacteria and only after an aerobic bacteria while in the group without nasalpolyps there was a predominance of an aerobic bacteria in relation to aerobic Gram-negative bacteria.
Conclusion: As recently suggested by several authors, nasalpolyps may not beast age of chronic rhinosinusitis but a distinct disease entity. The existence of distinct microbial flora eventually comes to corroborate this hypothesis.
Keywords: Chronic rhinosinusitis; Polyposis; Microbiology
Introduction
Chronic rhinosinusitis (with or without nasalpolyps) is defined as an inflammation of the nose and paranasals in uses that persists beyond 12weeks, characterized with two or more of the following symptoms: nasal obstruction/congestion, anterior and/or posterior rhinorrhea, localized pain or facial pressure and reduction/loss of smell. In addition to, atleast two of these symptoms, there must be polyps/purulent rhinorrhea discharge from the middle meatus/ Oedema in endoscopic examination. In the CT scan there must be pathological alteration of the sinuses or atleast of the osteo-meatal complex [1,2]. Despite of chronic rhinossinusitis prevalence, its etiology remains a target of great discussion and research.
Chronic rhinosinus it is the major pathology and nasalpolyps are considered a sub-group of this entity. Currently there are many authors who advocate that they consist in two different entities, based mainly on the difference of inflammatory markers present in patient with and without polyps. This classification change may ultimately lead to different ways of approach and treat the patients [3,4].
The origin of microorganisms in the nasal mucosa may eventually cause sinus it is. It is broadly defined in the literature the type of microorganisms present in chronic rhinosinusitis. There are also some authors that, given the known difference between the rhinosinusitis subgroups (with and without polyps), have compared the microbiological flora between these two groups [5-7].
Objectives
This study aims to evaluate and compare the microbial flora in patients with chronic rhinosinusitis with and without nasalpolyps.
Methods
This was a prospective study in the department of otorhinolaryngology at Centro Hospitalardo Porto. The sample size was of 110 patients with chronic rhinosinusitis and with surgical treatment indication. The study took place between January 2010 and March 2014. It was validated by the hospital ethics committee and all patients gave informed consent.
The authors excluded patients under 18 years old, patients with cysticfibrosis, immunodeficiencies, malignant disease, congenital mucociliary disease, fungal disease and vasculitis or granulomatous diseases.
These patients were classified as with and without nasalpolyps by endoscopic examination, and the score Lund-McKay was evaluated.
At the beginning of each surgical procedure, a sample of mucosa of the osteomeatal complex region was collected; on the side with the highest score Lund-McKay. All biopsies were collected in asterile container then inoculated into the culture media with in1-4h of collection.
For aerobic culture, biopsies were in oculated in MacConkeyagar, Chocolateagar, Sabouraudagar and Cooked meat broth. The media (plates and broth) were incubated at 35ºC (Chocolate agar in a 5% carbon dioxide environment). The primary plates and broth were examined daily for atleast two days for any microbial growth, the broth was sub culture at 48h to MacConkey agar and Chocolate agar and examined daily for four more days. Sabouraud was examined daily for seven days for any fungal growth. For an aerobic culture, biopsies were in oculated in Columbia blood agar base with hemin, G.N.an aerobes medium and N.E. an aerobes medium. These media were incubated an aerobically at 35ºC and evaluated twice a week for any microbial growth, for atleast seven days.
Data analysis was performed using IBM-SPSS Statistics version 23. Thechi-square test or the Fisher´s exact test (when appropriate) was used to determine whether the prevalence of the different types of bacteria was significantly different between the two groups of patients, with polyps (CRSwNP) and without polyps (CRSsNP).
Results
37 different bacteria were identified. In 4patients with CRSsNP and in 15 patients with CRSwNP, more than one type of bacteria was isolated. Analyzing all patients (n=110) there was a predominance of colonization/ infection by Gram-positive bacteria (65,45%), with predominance of Staphylococcus (47,27%). 26,36% (n=29) of the samples show edno cultural growth of microorganisms and were classified as sterile. In 18,82% there was growth of aerobic Gram-positive bacteria. The authors present in the table the Grampositive and Gram-negative an aerobic bacteria and also the total of an aerobic bacteria identification. They identified an aerobic bacteria in 18, 18% of the patients (Table 1 & 2), (Figure 1).
Table 1: Microbiological identification in patients with CRS in 15patients, more than 1 agent was identified.
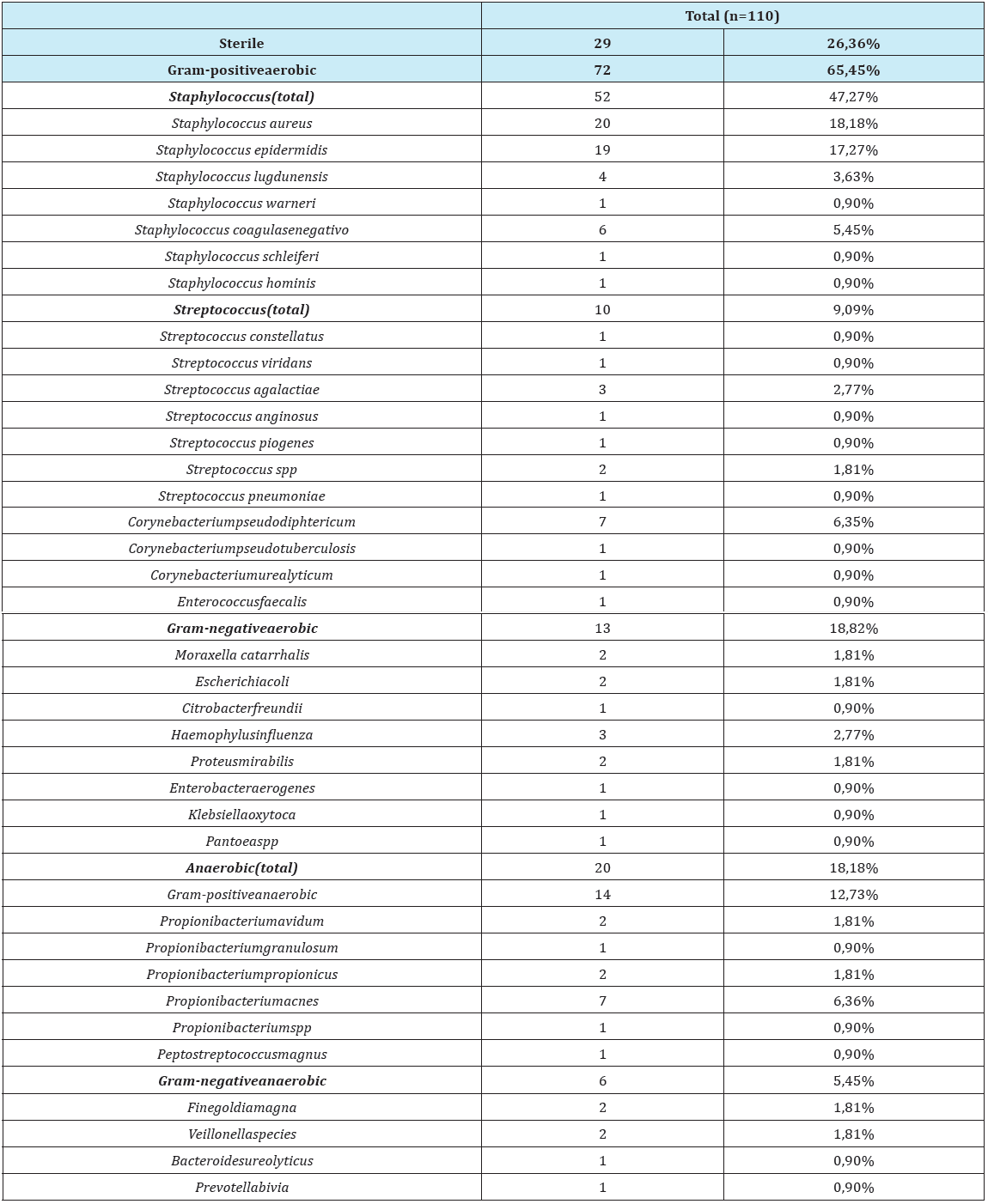
Table 2: Comparative analysis of microbial flora obtained by other authors and present study.
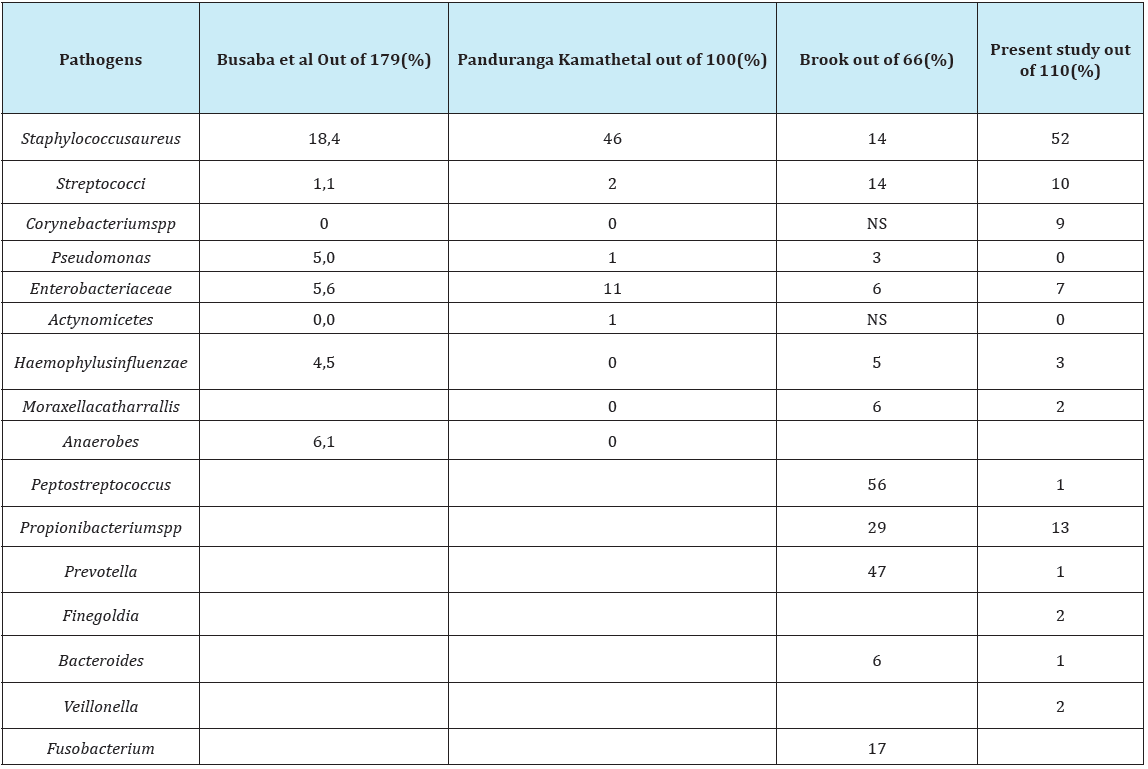
Table 3: Microorganisms frequency in CRSwNP and CRSsNP.
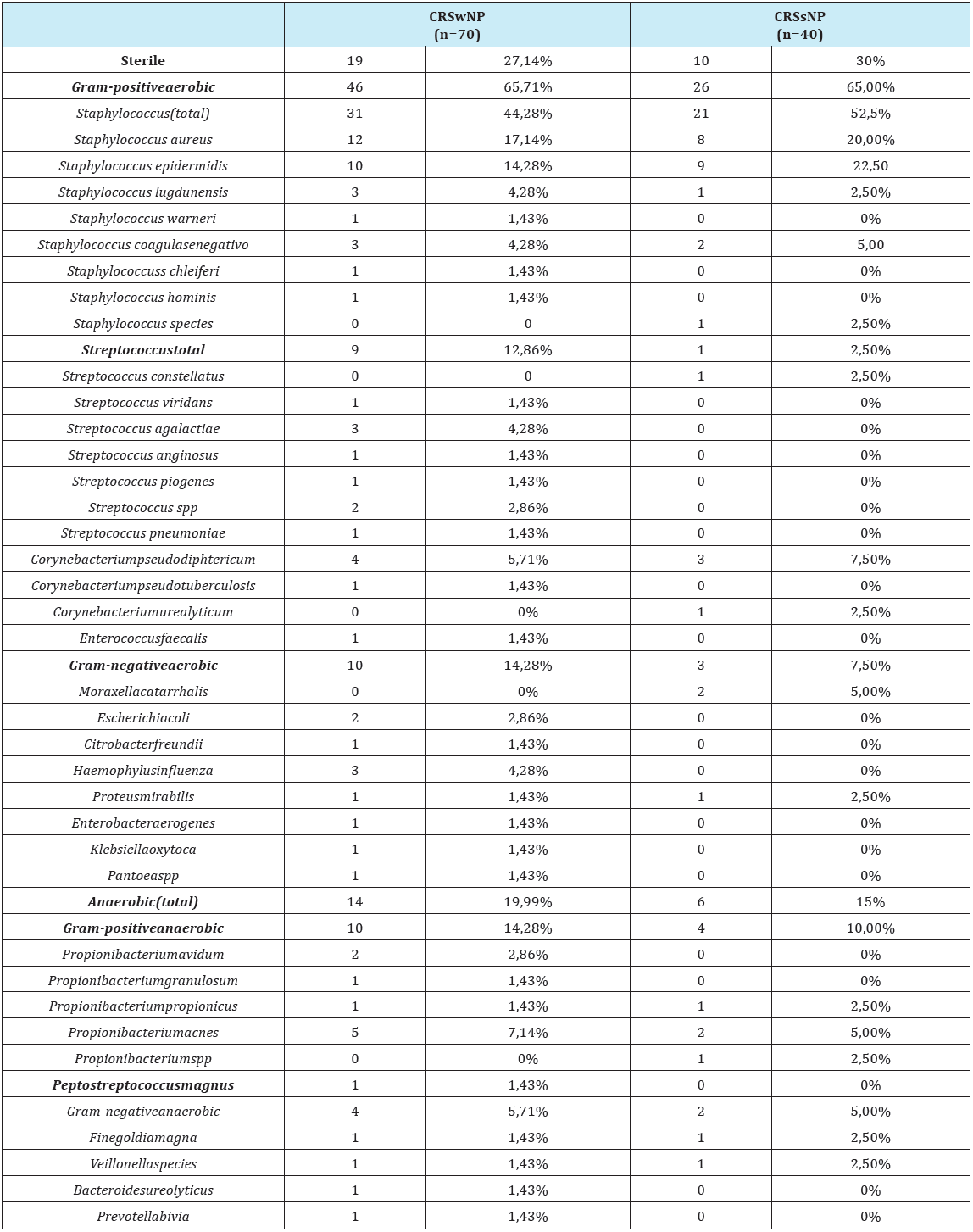
Figure 1: Results in patients with CRS.
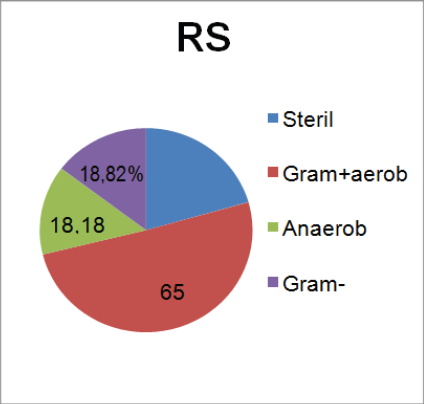
Figure 2: Results in patients with CRSwNP.
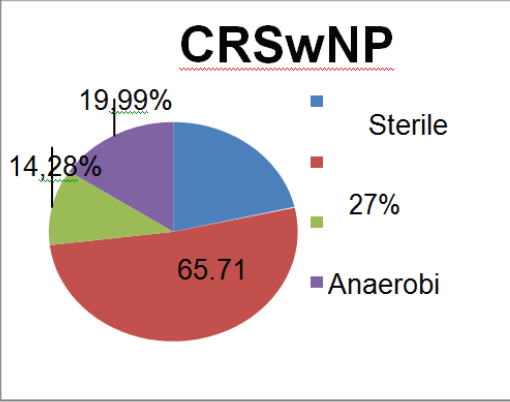
Figure 3: Results in patients with CRSsNP.
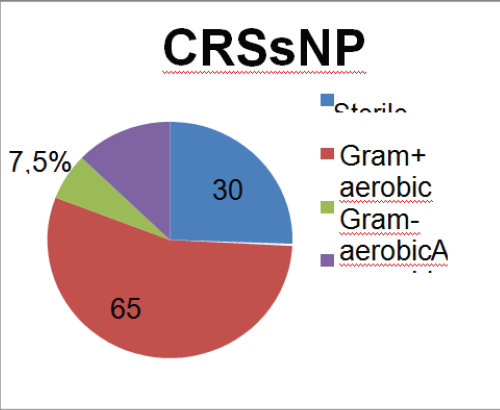
Evaluating separately the sub-group with polyps (CRSwNP) and without polyps (CRSsNP), the authors found that the prevalence of aerobic Gram-positive bacteria persists (predominantly Staphylococcus). However, in both groups the an aerobic stake these cond place in the rank, followed by the Gram-negative aerobic bacteria. The comparison between the two sub-groups show that the aerobic Gram-negative bacteria are more frequent in the CRSwNP group than in the CRSsNP group, although not statistically significant (p=0.229, one sided Fisher´ sex act test) (Table 3), (Figure 2 & 3).
Discussion
One can find several studies concerning the bacterial identification in patients with chronic rhinossinusitis [5,7-15]. However, most of these did not search for an aerobic bacteria. In our study, we have found more positive identifications of an aerobic bacteria than aerobic Gram-negative bacteria. We isolated 10 different an aerobic bacteria. There so, a study that does notinclude an aerobic culture is not complete. It is not worthy that we found an aerobic bacteria in both groups. The major difference between the two groups was the prevalence of the aerobic Gram-negative bacteria that was twice higher in the CRSwNP group and, although not statistically significant (p=0.229), one sided Fisher´ sex act test, it seems that, in a larger population, the significance would be achieved.
Conclusion
Chronic rhinosinusitis, as a single entity, is very heterogeneous and their phenotypic differentiation in chronic rhinossinusite with and without polyposis is consensual. Their distinct peculiarities, despite the phenotype, are related with its inflammatory mediators. The existence of different microbiological flora can be another distinguishing factor. Extrapolation of these differences leads to the need to address these subtypes as distinct clinical entities, which already is suggested by different authors. Perhaps this will conduct to different treatments in the future.
References
- Lund VJ, Mackay IS (1993) Staging in rhinosinusitus. Rhinology 31(4): 183-184.
- Bhattacharyya N (2002) The role of infection in chronic rhinosinusitis. Curr Allergy Asthma Rep 2(6): 500-506.
- Huvenne W, van Bruaene N, Zhang N, van Zele T, Patou J, et al. (2009) Chronic rhinosinusitis with and without nasal polyps: what is the difference? Curr Allergy Asthma Rep 9(3): 213-220.
- Rudack C, Sachse F, Alberty J (2004) Chronic rhinosinusitis--need for further classification? Inflamm Res 53(3): 111-117.
- Brook I (2005) Microbiology and antimicrobial management of sinusitis. J Laryngol Otol 119(4): 251-258.
- Cain RB, Lal D (2013) Update on the management of chronic rhinosinusitis. Infect Drug Resist 6:1-14.
- Liu Q, Lu X, Bo M, Qing H, Wang X, et al. (2014) The microbiology of chronic rhinosinusitis with and without nasalpolyps. Acta Otolaryngol 134(12): 1251-1258.
- Al-Shemari H, Abou-Hamad W, Libman M, Desrosiers M (2007) Bacteriology of the sinus cavities of assymptomatic individuals after endoscopy sinus surgery. J Otolaryngol 36(1): 43-48.
- Bhattacharyya N (2005) Bacterial infection in chronic rhinosinusitis: a controlled paired analysis. Am J Rhinol 19(6): 544-548.
- Araujo E, Palombini BC, Cantarelli V, Pereira A, Mariante Al (2003) Microbiology of middle meatus in chronic rhinosinusitis. AmJ Rhinol 17(1): 9-15.
- Ragab A, Clement P, Vincken W, Nolard N, Simones F (2006) Fungal cultures of different parts of the upper and lower airway sin chronic rhinosinusitis. Rhinology 44(1): 19-25.
- Klossek JM, Dubreuil L, Richet H, Richet B, Sedallian A, et al. (1996) Bacteriology of the adult middle meatus. J Laryngol Otol 110(9): 847- 849.
- Gold SM, Tami TA (1997) Role of middle meatus aspiration culture in the diagnosis of chronic sinusitis. Laryngoscope 107(12 Pt 1): 1586-1589.
- Vogan JC, Bolger WE, Keyes AS (2000) Endoscopically guided sinonasal cultures: a direct comparison with maxillary sinus aspirate cultures. Otolaryngol Head Neck Surg 122(3): 370-373.
- Kamath MP, Shenoy SV, Mittal N, Sharma N (2013) Microbiological analysis of paranasal sinuses in chronic sinusitis–A south Indian coastal study. Egyptian Journal of Ear, Nose, Throat and Allied Sciences 14(3): 185-189.
© 2017 José Gameirodos Santos, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






