- Submissions
Full Text
Degenerative Intellectual & Developmental Disabilities
Case History of a Novel Treatment Approach for Congenital Cardiomyopathy due to Carnitine Transporter Deficiency
Arun Mukherjee*, Vinod Sharma, Indrajit Neil Roy and Meena Gupta
National Heart Institute, India
*Corresponding author: Arun Mukherjee, Sr. Consultant in Medicine, National Heart Institute, Delhi, India; Medical Research Director, UDAAN for the Disabled, Lajpatnagar-4, New Delhi, India
Submission: September 26, 2017; Published: December 19, 2017

Volume1 Issue November 2017
Abstract
This is a case study of a novel experimental therapy approach in a rare treatment non-responsive potentially fatal Carnitine Transporter Deficiency Congenital Cardiomyopathy. The child's LVEF was down to about 24% at presentation. As a last resort, we tried out a new multidisciplinary therapy approach. It restored his treatment response and raised his LVEF to a persistent level of 80 to 84% as followed up for last two years. Three of his similarly affected siblings: two brothers and one sister, had earlier succumbed in spite of optimum Levo-Carnitine supplement (the only approved treatment) as provided by one of the most prestigious Medical Institutes of his country
Keywords: Cardiomyopathy; Carnitine deficiency; Fatty acid transporter; Hyperbaric oxygen therapy
Introduction
Carnitine deficiency is also known as carnitine transport deficiency. It is an inborn error of fatty acid transport caused by defect in the transporter who is responsible for moving the plasma membrane across the plasma. Carnitine play an important role in amino acid metabolism [1]. The clinical features of carnitine deficiency with patient show non-specific presentation such as low plasma carnitine level in the blood. The incidence of patients with newborn screening has allowed early intervention and treatment. Its treatment includes high dose carnitine supplementation which must be continued till life [2]. Carnitine deficiency results from inadequate intake of or inability to metabolize the amino acid carnitine. It can cause a heterogeneous group of disorders. Muscle metabolism is impaired, causing myopathy, hypoglycemia, or cardiomyopathy. Infants typically present with hypoglycemic, hypoketotic encephalopathy. Most often, treatment consists of dietary l-carnitine.
Case History
Patient
QAB, 16 years, Male.
Diagnosis
Carnitine transporter deficiency Type-2 causing congenital cardiomyopathy with severe Left Ventricular Failure
History
QAB was diagnosedat Aga Khan University (AKU) Hospital, Karachi, as a progressive case of Congenital Cardiomyopathy at the age of 2 years due to of Carnitine Transporter Deficiency Type-2. He had been on heart failure medications and high dose carnitine as per prevailing medical practice, which had helped to prolong his life till 16 years. He exhibited slowly deteriorating heart failure, with LVEF fluctuating every a few months, causing relapses and remissions, in spite of optimum recommended carnitine supplementation plus heart failure therapy. QAB came here in the beginning of 2015 with LVEF 30%, and breathlessness on climbing 2 flights of stairs.
Family history
Out of six brothers and sisters, one sister and 2 brothers, affected by the same disorder, died by teenage due to heart failure due to the above disease, in spite of available optimum doses of recommended therapy. One younger brother and one sister seem to be unaffected till date.
Regenerative therapy given by us in beginning of 2015
QAB was continued with earlier medications, and regulated low salt diet as per standard heart failure regimen initiated at AKU Hospital, to which he was showing features of non-response as did his 2 brothers and 1 sister and who had earlier succumbed to the disease.
On our part, we first added Hyperbaric Oxygen Therapy (HBOT), using 1.5 ATA pressure with 100% Oxygen to breathe, for 90 minutes per session to improve microcirculation. After 20 sessions of HBOT, we transplanted site-specific, intra-site, specific dose of a specific type of stem cell obtained from allogenic Wharton's Jelly, under the provisions of Helsinki Protocol Para 35 for treatment failure case using experimental therapy. This was followed up by 20 more HBOT sessions before he went back home. QAB was advised regular follow up and a booster dose at 1 year, to ensure better long term prognosis, till a definitive and proven therapy is approved for his condition.
Outcome of first round of therapy
Left ventricular ejection fraction (LVEF) went up from 30% to 35% at discharge from India: National Heart Institute Hospital, Delhi, in February 2015. LVEF was periodically assessed in his home-country: Aga Khan University Hospital, Karachi. He showed gradual improvement to reach an LVEF of 50% by mid-2015.
Outcome of second round of therapy
LVEF remained stable without relapse between 50 to 55% when we reviewed his case a year later at Delhi in January 2016 during his first annual checkup. He received a booster therapy consisting of HBOT 20 sessions followed by repeat stem cell transplantation followed by 20 more HBOT as given before. After full courses, LVEF had remained stable in India at 50 to 55%. His echocardiogram still showed some hypertrophic and dilated Left Ventricle and Atrium as before (Figure 1).
Figure 1: Pre-Regenerative Therapy ECG.
Discharge advice after second round of therapy
a. QAB was strongly advised to maintain drugs advised regulated salt intake, balanced diet and life style as recommended by his Pediatric Cardiologist at AKU Hospital in his home town.
b. Beta blockers, as given by his treating Pediatric Cardiologist.
c. We suggested replacing his betablocker with carvedilol, since it also reduces intra-hepatic BP significantly to reduce intrahepatic portal venous pressure and enhance liver recovery, which was border line deranged in QAB’s case.
d. Any symptomatic therapy if needed provided it was permissible in heart-failure.
e. L-Carnitine supplementation as per internationally accepted recommendations, as also prescribed by his Pediatric Cardiologist.
f. Physical activity to be regulated from time to time, based on clinical features, effort tolerance and local weather, as allowed by his Cardiologist.
g. Next follow up due in January 2017.
h. If anything medically new arises, he would be advised accordingly.
i. Regular follow up with his pediatric cardiologist and assessment of Echocardiogram and Liver Function Tests and sending us a copy of the reports.
Follow up echocardiogram January 2017
LVEF done at AKU Hospital showed improved cardiac function an LVEF of 84% which is maintained. The child is enjoying normal physical activity for his age without overstraining it.
Discussion
Carnitine Transporter Deficiency induced cardiomyopathy is a rare autosomal recessive genetic disorder, characterized by plasma membrane carnitine transport defect, impairing uptake and oxidation of long chain fatty acids at the mitochondrial level [3]. This may cause edema, sometimes therapy resistant heart failure, metabolic acidosis, hypoglycemia, muscle weakness. Investigations show ECG with giant T waves with prolonged QT intervals, and subnormal blood carnitine levels (Figure 2).
Figure 2: Pre-Procedure PFT at AK Univ. Hosp. Karachi.
As per Fernando Scaglia et al. [4], Carnitine (beta-hydroxyl- gamma-trimethyl-amino-butyric acid), which is a naturally occurring hydrophilic amino acid derivative, produced endogenously in the kidneys and liver and derived from meat and dairy products in the diet, cannot easily enter mitochondria and get oxidized for energy production and formation of ketones for brain function. Carnitine plays an essential role in the transfer of long chain fatty acids into the mitochondria for beta-oxidation [5].
It helps to decrease the number of acyl residues conjugated with coenzyme A (CoA) and increasing the ratio between free and acylated CoA. When its level becomes less than 10 to 20%, the condition becomes symptomatic due to reduced entry of fatty acids and reduced oxidation by mitochondrial activity. Accumulation of free CoA inside the mitochondria affects other pathways like Krebs cycle [6], pyruvate oxidation [7], amino acid metabolism, mitochondrial and peroxisomal beta oxidation. Organ dysfunction may involve cardiac/skeletal muscle, liver and brain/CNS function. Sudden onset of tachy-arrhythmia of heart may lead to sudden death.
As per Koizumi A et al. [8] a Japanese study showed an incidence of 1 in 40,000 births whereas as per Scaglia, the incidence has been variably reported as 1:37,000 to 1:100,000 newborns in Australia and 1:15,500 in United Kingdom. The most common early manifestations are usually cardiomyopathy causing heart failure, tachy-arrhythmias, sudden death or hypoglycemic hypoketotic (due to decreased and defective mitochondrial function) encephalopathy. The disease is race and sex independent [9]. Meanwhile, the old drugs that the boy was continuing to use, were continued as before, appears to have converted the boy from a non-responder to L-Carnitine supplementation in to a responder, unlike his two brothers and one sister who had succumbed on only standard approved therapy given earlier.
Management
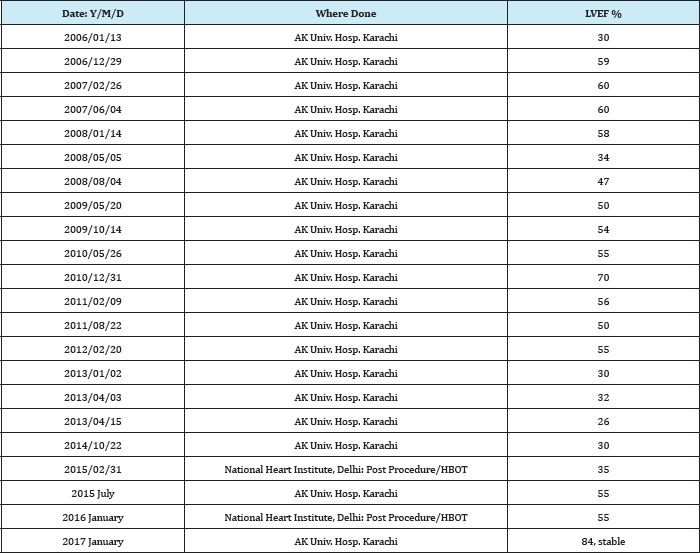
Lifelong treatment with very high dose supplementation with L-carnitine and avoidance of fasting or prolonged exercise are required. Periodic echocardiogram, ECG and plasma carnitine level monitoring should be done. Oral L-carnitine is used, typically at a dose of 100-400mg/kg/day, in two to three divided doses. This regimen has been found to be highly beneficial in majority of cases of Carnitine Transporter Deficiency. However, some cases still succumb to heart failure and sudden death, as happened with the two brothers and one sister of QAB, with him also gradually progressing along the same degenerative path in spite of using heart failure therapies supplemented by recommended L-Carnitine therapy under the guidance of his Cardiologists (Table 1).
Table 1: LVEF data from Echo-Cardiogram record.
This being a treatment failure case of carnitine transporter deficiency induced cardiomyopathy, who appeared to be in the last lap of his lifespan, we decided to use desperate measures. Hyperbaric Oxygen Therapy [10] is a means to provide a patient 100% oxygen under higher than normal pressure inside a pressure chamber. It has been shown during the last more than 4 decades of clinical use (other than older history of therapy of "bends" in deep sea divers who come up too fast), to produce the following relevant benefits [11]:
a. Accelerate and improve the cellular repair mechanisms
b. Improve mitochondrial function and cellular metabolism
c. Improve axonal regeneration and myelinisation?
d. Increases neuroplasticity by reactivating neurons and glial cells in vegetative state
e. Increases the amount of circulating stems cells
f. Decreases apoptosis
g. Increases angiogenesis
In January 2015, we chose to use HBOT prior to use of stem cell therapy [12] based on the work of Diane Barbosa et al. [13] in early 2000s. They showed that stem cell therapy preceded and followed by 20 sessions of HBOT each time, gave a significantly greater benefit than either therapy alone. We chose to follow the same protocol. Our protocol uses a specific type of stem cell class, derived from a specific donor tissue, meeting safety, viability, sterility and other specificity criteria as per recommended guidelines. Our protocol also used a specific method of direct onsite transplantation method into the tissue(s) selected (heart as well as liver).
We achieved gratifying results, with LVEF improving from 30% to 35% post procedure. This appears to be a direct HBOT effect as it works quickly whereas stem cell mediated repair and regeneration often takes one year or more. His LVEF improved to 50 to 55% by July 2015, and normalising at 80 to 84% as of mid-2017, giving us hope. We have tasted some degree of success, but, based on one case study, it is difficult to quantify the degree of contribution by HBOT and stem cell transplantation. We hope to do more cases, if such cases are available.
Summary and Conclusion
This is a long term follow up record of a single case study of the rare Carnitine Transporter Deficiency Type 2 induced near fatal cardiomyopathy, after it had already taken the lives ofthree similarly affected siblings of his family (two brothers and one sister), in spite of recommended doses of carnitine supplementation and heart failure medications, managed by one of the best hospitals of their country. This child, one of four brothers and two sisters, came to us with LVEF of 30% at end 2014, and was stable two years later at an LVEF of 84%. His treatment non-responsive state, which took the lives of his three siblings earlier, has started responding to standard medications, and he leads a near normal life like any young teenager of his age.
We continued his heart failure strategies and carnitine supplementation as before, and added hyperbaric oxygen therapy and a specific protocol of stem cell transplantation, with a booster dose after one year. We did not observe any adverse reaction from our treatment protocol, even after more than 2 years. With data on only one seemingly successfully stabilised case, it is difficult to quantify the relative degree of help obtained by this previously non-responder case, from hyperbaric oxygen therapy and stem cell therapy given together. It is hard to come by an adequate number of such cases to be available at any one center, for doing a full-fledged clinical trial, given the rare nature of the disorder. Hence, going by this single safe and effective case study record, we propose to follow the same protocol for future cases if any, till we have more data.
References
- Lund AM, Joensen F, Hougaard DM, Jensen LK, Christensen E, et al. (2007) Carnitine transporter and holocarboxylase synthetase deficiencies in the Faroe Islands. J Inherit Metab Dis 30(3): 341-349.
- Lee NC, Tang NLS, Chien YH, Chen CA, Lin SJ, et al. (2010) Diagnoses of newborns and mothers with carnitine uptake defects through newborn screening. Mol Genet Metab 100(1): 46-50
- Cano A, Ovaert C, Vinaey-Saban C, Chabrol B (2008) Carnitine membrane transporter deficiency: A rare treatable cause of cardiomyopathy and anemia. Pediatr Cardiol 29(1): 163-165.
- Fernando Scaglia (2014) Carnitine Deficiency.
- Longa N, Amat C, Pasquali M (2003) The OVTN2 carnitine transporter and fatty acid oxidation. Membrane transporter disease, pp. 161-174.
- Kornberg H, Williamson DH (1984) Hans Adolf Krebs. 25 August 190022 November 1981. Biographical Memoirs of Fellows of the Royal Society 30: 350-385.
- Yu-Ling Y, Jonathan L, William BW (1992) Pyruvate oxidation by methanococcus spp. Arch Microbiol 158(4): 271-275.
- Koizumi A, Nozaki J, Ohura T, Kayo T, Wada Y, et al. (1999) Genetic epidemiology of the carnitine transporter OCTN2 gene in a Japanese population and phenotypic characterization in Japanese pedigrees with primary systemic carnitine deficiency. Hum Mol Genet 8(12): 22472254.
- Stanley, Charles A, Bennett, Michael J, Longo, Nicolo (2004) Plasma Membrane Carnitine Transport Defect. In: Scriver CW, Beaudet AL, Sly WS (Eds.), Metabolic and Molecular Bases of Inherited Disease (8th edn), McGraw Hill, New York, USA.
- Lerche CJ, Christophersen LJ, Kolpen M, Nielsen PR, Tr0strup H, et al. (2017) Hyperbaric oxygen treatment augments tobramycin effect in experimental staphylococcus aureus endocarditis. Int J Antimicrob Agents 50(3): 406-412.
- Jain KK (1996) Text Book of Hyperbaric Medicine.
- JJ Catherine, Tonseth KA, Utheim TP (2017) Cultured epidermal stem cell in regenerative medicine. Stem Cell Res Ther 8(15): 155.
- Barbosa D (2003) 3rd Int. Symposium on Cerebral Palsy and the Brain Injured Child. Fort Lauderdale, Florida, USA.
© 2017 Arun Mukherjee, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






