- Submissions

Full Text
Developments in Anaesthetics & Pain Management
Dexmedetomidine as a Sole Sedative Agent versus Propofol for Sedation during Upper and Lower Gastrointestinal Endoscopies
Alaa Ali Elzohry1*, Adnan Ahmed Ali2, Waleed Attia Hassan2 and Wael Abd Elgwad Elsewify3
1 Lecturer of Anesthesia, Assiut University, Egypt
2 Lecturer of Tropical medicine and Gastroenterology, Assiut University, Egypt
3 Department of Internal medicine, Aswan University, Egypt
*Corresponding author: Alaa Ali Elzohry, Department of Anesthesia ICU and Pain Relief, Assiut University, Egypt
Submission: July 12, 2018;Published: August 16, 2018

ISSN: 2640-9399 Volume1 Issue4
Abstract
Introduction and objectives: Diagnostic and therapeutic procedures recently are done in gastroenterology setup as a part of fast-track concept. A major volume of gastrointestinal procedures are performed routinely on daycare basis under sedation as upper and lower GIT endoscopy. Many anesthetic agents used to provide sedation for these procures. Propofol, opioids, and midazolam form the backbone of the various regimes employed in the endoscopic suites all over the world. Dexmedetomidine is a pharmacologically active selective α 2-adrenergic receptor agonist. It was approved it in the intensive care unit (ICU) for sedation and analgesia for the duration of less than 24 hours. The aim of this study was to study efficacy and safety of Dexmedetomidine efficacy as sole sedating agent versus propofol for sedation during upper and lower GIT endoscopy.
Methods: This randomized controlled trial was carried out on 60 patients of either sex, aged 21-70 years of age undergoing upper and lower GIT endoscopy, with ASA I-II. Patients were randomly assigned into two groups, (30 patients in each group).
Dex group: Sedation was induced by loading dose of (dexmedetomidine 1μg/kg) followed by infusion of (dexmedetomidine 0.8μg/kg /h) Propofol group: Sedation was initially started by bolus dose of 0.5mg/kg propofol IV Then, infusion was started at the rate of 50μg /kg/min. Upper and lower GIT endoscopies were carried out in the usual standard manner for all patients, then patients were discharged to PACU after attaining an Aldrete Recovery Scale Score of 9-10 Time taken to achieve this score was recorded. The patient’s vital signs, Respiratory complications, VAS score for pain measurement, PONV, and any other adverse events were recorded.
Results: There was significant decrease in (HR and MAP) but not respiration rate (RR) and SpO2, in (Dex group) during the procedure and early post-operative (P. value 0.000**). But during the remaining of post-operative periods (HR and MAP) were comparable. VAS pain scores in both groups were decreased in comparable manner at all measured time points. But complications (atthythmia, air way obstruction, nausea, and vomiting) was significantly increased in Propofol group (P. value 0.001**). Mean time to achieve RSS 3-4 was 6 (±1.5) min in Dex group versus 9 (±1.9) min in Propofol group (P< 0.005) and to achieve an Aldrete Recovery Scale Score of 9-10 was 8 (±2.1) min in Dex group versus 6 10 (±1.6) min in Propofol group (P< 0.029).
Conclusion: In conclusion, there is evidence to support dexmedetomidine as a potential sole sedative agent in small diagnostic and therapeutic procedures like GIT endoscopies, our study support these evidences and although dexmedetomidine resulted in longer onset and recovery, more side effects but sufficient levels of sedation and analgesia are good advantages to use it as sole sedating agent.
Keywords: Dexmedetomidine; Sedation; Propofol; GIT endoscopy
Background and Objectives
Sedation level can be divided into minimal, conscious, deep, and general anesthesia. A sedative decreases the level of consciousness, allows a patient to withstand and tolerate a painful procedure (whether or not in combination with a local anesthetic), and minimizes discomfort and memory of the procedure. Minimal-tomoderate sedation is generally sufficient to maintain spontaneous respiration and protect airway reflexes [1-3]. Diagnostic and many therapeutic procedures recently are done in gastroenterology setup as a part of fast-track concept. A major volume of gastrointestinal procedures are performed routinely on daycare basis under sedation as upper and lower GIT endoscopy [4].
Criteria of an ideal sedative agent include; rapid onset has a predictable clinical effect and should be easily titrable [5]. Respiratory and hemodynamic stability are two factors of paramount importance for procedural sedation. Many anesthetic agents used to provide sedation. Propofol, opioids, and midazolam form the backbone of the various regimes employed in the endoscopic suites all over the world [6-8].
Propofol, the most commonly used sedating agent, is a potent hypnotic agent with rapid onset of action and rapid recovery. But dose dependent cardiac and respiratory depression with inadequate analgesic action; represent the common adverse effects observed with it [9] Dexmedetomidine (DEX) -dextroisomer of medetomidine- is a pharmacologically active selective α 2-adrenergic receptor agonist. It was introduced in the year 1999, when the US Food and Drug Administration approved it in the intensive care unit (ICU) for sedation and analgesia for the duration of less than 24 hours [10-12]. The aim of this study was to study efficacy and safety of Dexmedetomidine efficacy as sole sedating agent versus propofol for sedation during upper and lower GIT endoscopy.
Patients and Methods
After approval of local ethical committee of Assiut University and written informed consent from patients, this randomized controlled trial (RCT) was carried out on 60 patients of either sex, aged 21- 70 years of age undergoing upper and lower GIT endoscopy, with American Society of Anaesthesiologist (ASA) Grade I, II. Patients who had ASA physical status Grade III, VI, baseline SpO2 < 90%, patients who had difficulty in communication, who refused the study, patients allergic to the studied medications, morbidly obese patients, patients with chronic obstructive pulmonary disease, complicated airway, and pregnant patients were excluded.
Patients mostly presented by; haematemsis, bleeding per rectum, melena and chronic diarrhea. One day before endoscopy, data were collected as; demographic data, medical, physical fitness and routine laboratory investigations. On the arrival of patient in endoscopy unit, IV access was inserted and secured and 0.03mg/ kg midazolam was given as sedation and Ringer Lactate drip was started. During endoscopy, vital parameters such as (HR, mean arterial pressure MAP and oxygen saturation SPO2) were recorded as baseline and every 5min until the completion of the procedure.
Patients were randomly assigned into two groups, (30 patients in each group), as following; opaque sealed envelopes containing a computer generated randomization schedule; the opaque envelopes were sequentially numbered and were opened immediately before application of anesthetic plan.
Dex group (no. =30 patients)
Sedation was induced by loading dose of (dexmedetomidine 1μg/kg slowly infused over 5 minutes) followed by infusion of (dexmedetomidine 0.8-1.2μg/kg /h).
Propofol group (no. =30 patients)
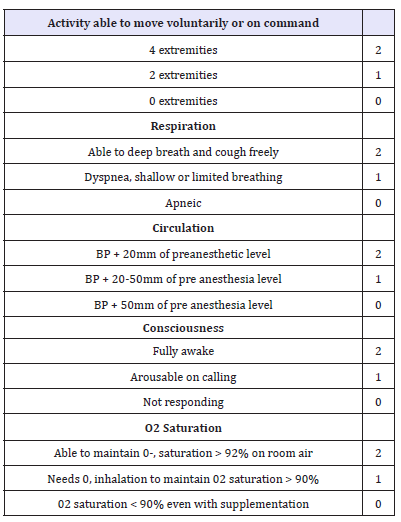
Sedation was initially started by bolus dose of 0.5mg/kg propofol IV over 3 minutes then, infusion was started at the rate of 50-100μg/kg/min. The level of sedation was assessed at 1-3min intervals in both group, and the infusion rate was adjusted accordingly to achieve a Ramsay Sedation Scale (RSS) score of 5 (Table 1) [13]. Upper and lower endoscopies were carried out in the usual standard manner for all patients and after routine preparations. Infusion was discontinued at the end of the procedure, and the recovery time was recorded and calculated as the time from discontinuation of infusion of the study drug till achievement of RSS score of 3 then patients were discharged to PACU after attaining an Aldrete Recovery Scale Score of 9-10 (Table 2) [14]. Time taken to achieve this score was also recorded.
Table 1:Ramsay sedation scale.
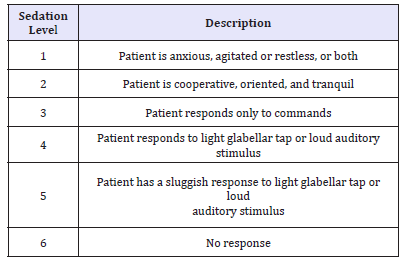
Table 2:The modified aldrete scoring system
Any respiratory depression (desaturation (SpO2 dropped to < 90% or cessation of respiration for more than 15 seconds), were recorded, and managed by supporting the airway and ventilation. Also hypotension was defined as systolic blood pressure < 85mmHg and was treated with IV fluid plus IV ephedrine 0.1mg/kg. Bradycardia was defined as HR slower than 50 beats/min and was treated by atropine 0.01mg/kg. All patients were followed up for 12 hours for the following parameters; VAS scores for pain measurement, which was recorded at 0, 1, 2, 4, 6, and 12 hours. Any other adverse events (e.g. nausea and vomiting, arrhythmia, airway obstruction and laryngeal spasm) were recorded and were managed accordingly. The patient’s vital signs were assessed at regular intervals.
Statistical analysis
Data analysis: Statistical analysis was carried out using SPSS® version 21 software. Normality distribution of continuous data was tested. Data were expressed as number, percentage, mean and standard deviation. Chi-square test was used in order to compare qualitative variable among studied groups. Independent t- test was used to compare quantitative variables between the two studied groups. P Value < 0.05 was considered statistically significantly. Sample size estimation: The sample size included all eligible patients admitted to the GIT endoscopy unit from August 2017 to March 2018 who were consecutively enrolled to detect a 20% improvement in Achievement of modified Aldrete score of 9-10 (average standard deviation 0.8cm) with a power of 0.8. To account for the multiple outcomes and dropouts we increased the sample size to 24 patients per group.
Results
This study involved two groups of patients who underwent diagnostic and therapeutic upper or lower GIT endoscopy, DEX group (n=30) and the Propofol group (n=30). The demographic data, the patient’s characteristics and baseline vital signs between the two groups were statistically insignificant (Table 3). There was significant decrease in (HR and MAP) but not respiration rate (RR) and SpO2, in (Dex group) during the procedure and early post operative (P. value 0.000**) (Figure 1-3). But during the remaining of post operative periods (HR and MAP) were comparable (Table 4).
Figure 1:Intra-operative heart rate (HR) (beat/ min) data are expressed as mean ± SD. At 0 and 1, hours MAP= mean arterial pressure (mmhg), HR=heart rate (beat per minutes). h=hour interval Group D: Dexmedetomidine group P. value < 0.05 considered statistically significant. There was significant difference in intra operative periods being decreased in group Dex in comparison to Propofol group.
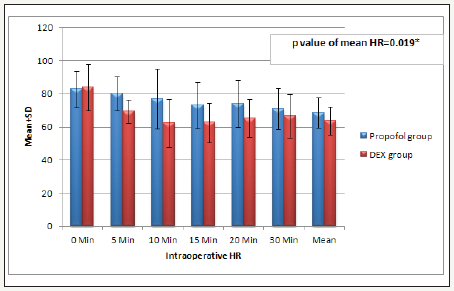
Table 3:Demographic data of the studied groups Data expressed as (Mean±SD) and number (%) DEX group: Dexmedetomidine group. P. value< 0.05 considered statistically significant. There was no significant difference between both groups.
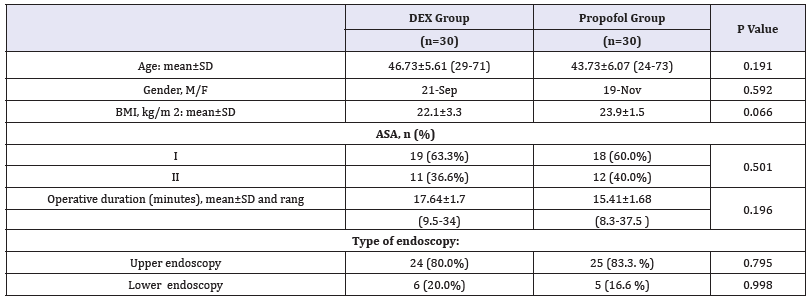
Figure 2:Intra-operative mean arterial pressure (MAP) mmHg Data are expressed as mean±SD. At 0 and 1, hours MAP= mean arterial pressure (mmhg), HR=heart rate (beat per minutes). h=hour interval Group D: Dexmedetomidine group P. value < 0.05 considered statistically significant. There was significant difference in intra operative periods being decreased in group Dex in comparison to Propofol group.
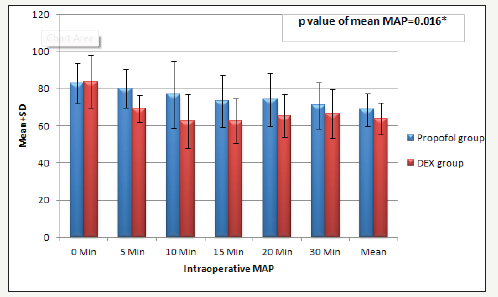
Figure 3:Intra-operative respiratory rate and saturation O2 Data expressed as (Mean±SD) and number (%) Dex group: Dexmedetomidine group. There was no significant difference between the two groups.
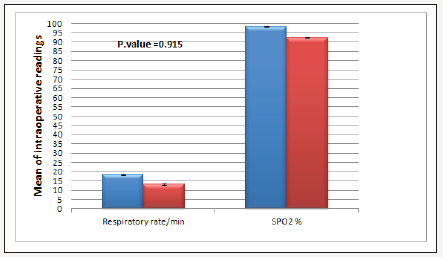
Table 4:Post-operative MAP and HR Data are expressed as mean±SD. At 0 and 1, hours MAP= mean arterial pressure (mmhg), HR=heart rate (beat per minutes). h=hour interval Group D: Dexmedetomidine group P. value < 0.05 considered statistically significant. There was significant difference in early post operative periods being decreased in group Dex in comparison to Propofol group.
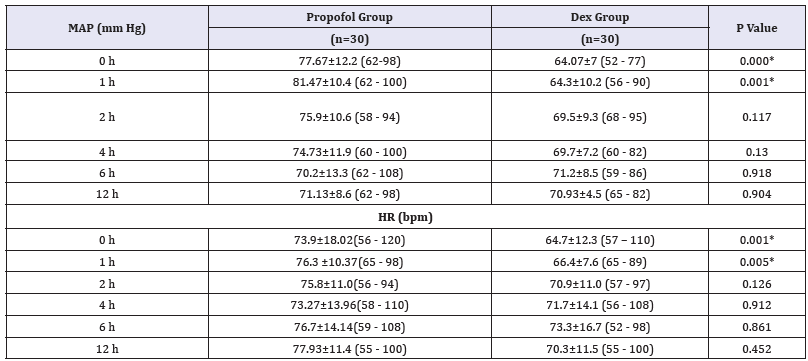
Figure 4:Post endoscopy complications Data are expressed as percentage, P. value < 0.05 considered statistically significant, Dex group: Dexmedetomidine. There was significant difference between the two groups.
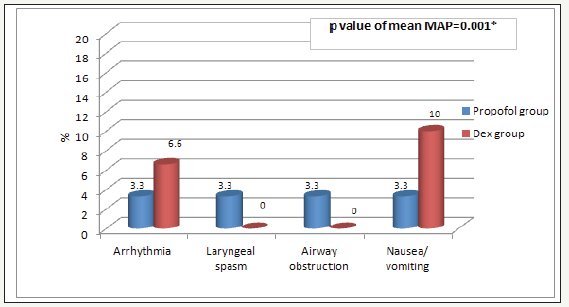
Figure 5:Flow diagram of patients through the study.
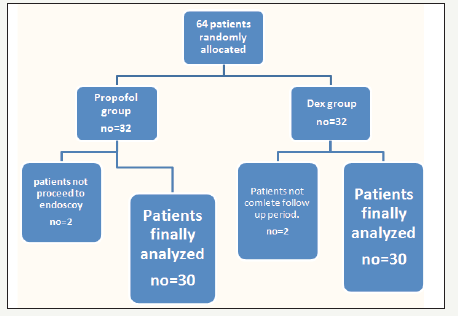
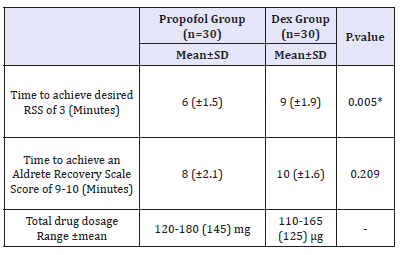
VAS pain scores in both groups were decreased in comparable manner at all measured time points (Table 5). Complications (laryngeal spasm and air way obstruction,) were significantly increased in Propofol group but nausea, vomiting and arrhythmia were decreased in Propofol group (P. value 0.001**) (Figure 4). Mean time to achieve RSS 3-4 was 9 (±1.9) min in Dex group versus 6 (±1.5) min in propofol group (P< 0.005) as time of onset of action of Dex was 2 minutes and to achieve an Aldrete Recovery Scale Score of 9-10 was 10 (±1.6) min in Dex group versus 8 (±2.1) min in Propofol group (P< 0.029) (Table 6) (Figure 5).
Table 5:Pain VAS scores during the postoperative 12 hours Data are expressed as median (range) VAS=visual analogue scale, h=hour. P. value< 0.05 considered statistically significant. Dex group Dexmedetomidine group P. value< 0.05 considered statistically significant. There was no significant difference between the two groups.
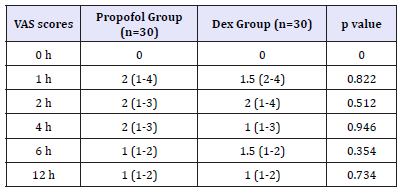
Table 6:Time to achieve; desired RSS of 3, and an Aldrete Recovery Scale Score of 9-s10 Data are expressed as mean ± SD, P. value< 0.05 considered statistically significant. Dex group: Dexmedetomidine group. There was significant difference between the two groups regarding Time to achieve; desired RSS of 3.
Discussion
During procedures like gastro-endoscopies, sedation is very important to enhance the comfort level of the patients, relief their anxiety associated with the procedure and facilitates patient cooperation and comfort level [15]. This prospective randomized controlled trial aimed to compare the efficacy and safety of IV dexmedetomidine versus propofol, for sedation during gastroendoscopies. The dose regimens of both drugs used in our study were similar to that used by many previous studies [16,17] was used in our study continuous infusion technique to maintain a steady state sedation level. Our results were as following, dexmedetomidine provides good level of sedation, tolerable side effects with longer recovery without significant decreased in hemodynamic parameters when compared to Propofol and both agents provide comparable levels of analgesia.
Dexmedetomidine was compared to propofol in many previous studies; as Anterior segment ophthalmic surgery, Fibreoptic nasotracheal intubation, Minor oral surgery and Septoplasty. All these procedures are minor and the results were In favor of propofol regaring level of pain by VAS and patients satisfaction [18-21]. Propofol which is a phenol derivative, with short duration of action administered as sedative and hypnotic agent. It has been used frequently over the past two decades as a sedative agent for endoscopic procedures. But unfortunately, propofol may cause deep sedation and dangerous side effects that need cardiopulmonary support [22].
But with the use of dexmedetomidine, hemodynamics was less aeffected by the stressful periods during the procedure. Which is considered beneficial effect especially for the elderly patients undergoing painful procedures who could be potentially hypertensive or ischemic [23]. And also the analgesic effects of dexmedetomidine which is mediated by α2 - alpha 2 adrenergic receptors present on the neurons of superficial dorsal horn in lamina II, by inhibiting the release of nociceptive transmitters, namely substance P and glutamate [24].
Against our results, a previous study in which dexmedetomidine was used a sole agent for sedation during minor procedures as ERCP versus propofol and the results were, less satisfactory sedation than propofol, as most of the patients needed additional sedatives to achieve a sufficient sedation level [25]. We can attribute these findings due to the use of dexmedetomidine as a sole agent with a relatively small dose similar to those employed in intensive care for sedation and in anesthesia as an adjunct agent.
But confirming our study, a study compared dexmedetomidine versus propofol during electrophysiology study and demonstrated comparable sedation level with either drug. And mean arterial blood pressure and respiratory rates values were significantly better at 5, 15min in dexmedetomidine group [26]. We can explain the occurrence of hypotension and bradychardia, observed in group dexmedetomidine because of dexmedetomidine is a highly selective α-2 adrenergic agonist with sedative and analgesic properties. It causes sympatholysis and affects hemodynamic stability [27].
Sympatholysis occurs due to the activation of postsynaptic α2 adrenergic receptors that results in hypotension, and Bradycardia thus helps in attenuating the stress response leading to ideal sedation [28]. Although there is a higher incidence of side effects as PONV and air way obstruction in the propofol group compared to dexmedetomidine group, this procedures may be associated with more respiratory complications especially during endoscopic insertion and throughout procedure due to either deep sedation or even light sedation in presence of secretions and endoscopic manipulations [29]. Regarding drug combinations, another study compared dexmedetomidine/fentanyl versus propofol/nalbuphine in plastic surgery found that though HR, SBP and DBP decreased intraoperatively in both groups but these decreases were more evident in the dexmedetomidine group [30].
In conclusion, there is evidence to support dexmedetomidine as a potential sole sedative agent in small diagnostic and therapeutic procedures like GIT endoscopies, our study support these evidences and although dexmedetomidine resulted in longer onset and recovery, more side effects but sufficient levels of sedation and analgesia are good advantages to use it as sole sedating agent.
References
- American Society of Anesthesiologists Continuum of Depth of Sedation (2010) Definition of general anesthesia and levels of sedation/analgesia, pp. 11-29.
- Ghali A, Mahfouz AK, Ihanamaki T, El Btarny A (2011) Dexmedetomidine versus propofol for sedation in patients undergoing vitreoretinal surgery under sub-Tenon’s anesthesia. Saudi J Anaesth 5: 36-41.
- Gross J, Farmington CT (2002) American society of anesthesiologists task force on s analgesia by N-A: Practice guidelines for sedation and analgesia by non anesthesiologists. Anesthesiology 96(4): 1004-1017.
- Ljungqvist O, Thanh NX, Nelson G (2017) ERAS-value based surgery. J Surg Oncol 116(5): 608-812.
- Lichtenstein DR, Jagannath S, Baron TH, Anderson MA, Banerjee S, et al. (2008) Sedation and anesthesia in GI endoscopy. Gastrointest Endosc 68(5): 815-826.
- Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JT, et al. (2010) European society of gastrointestinal endoscopy, european society of gastroenterology and endoscopy nurses and associates and the European society of anaesthesiology guideline: non anesthesiologist administration of propofol for GI endoscopy. Endoscopy 42(11): 960- 974.
- Ahmed Ahmed, Alaa Ali ME (2018) Enhanced recovery after surgery: A better protocol for better outcomes. Archives of Anesthesiology 1(1): 1-7.
- Garewal D, Waikar P (2012) Propofol sedation for ERCP procedures: a dilemna? Observations from an anesthesia perspective. Diagn Нer Endosc 2012: 639190.
- Angsuwatcharakon P, Rerknimitr R, Ridtitid W, Kongkam P, Poonyathawon S, et al. (2012) Cocktail sedation containing propofol versus conventional sedation for ERCP: a prospective randomized controlled study. BMC Anesthesiol 9: 12-20.
- Wunsch H, Kahn JM, Kramer AA, Wagener G, Li G, et al. (2010) Dexmedetomidine in the care of critically ill patients from 2001 to 2007: An observational cohort study. Anesthesiology 113(2): 386-394.
- Taniyama K, Oda H, Okawa K, Himeno K, Shikanai K, et al. (2009) Psychosedation with dexmedetomidine hydrochloride during minor oral surgery. Anesth Prog 56: 75-80.
- Wang T, Ge S, Xiong W, Zhou P, Cang J, et al. (2013) Effects of different loading doses of dexmedetomidine on bispectral index under stepwise propofol target-controlled infusion. Pharmacology 91(1-2): 1-6.
- Ramsay MA, Savege TM, Simpson BR, Goodwin R (1974) Controlled sedation with alphaxalone-alphadolone. Br Med J 2(5920): 656-659.
- Aldrete JA, Kroulik D (1970) A Post anesthetic recovery score. Anesth Analg 49(6): 924-934.
- Qadeer MA, Vargo JJ, Khandwala F (2005) Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clin Gastroenterol Hepatol 3(11): 1049-1056.
- Elzohry AAM, Ali AAM, Shehata MR (2018) Ketamine versus Dexmedetomidine as adjunct agent to propofol for sedation during Endoscopic Retrograde Cholangiopancreatography (ERCP). Integr Anesthesiol 1(1): 01-07.
- Hasanein R, El-Sayed W (2013) Ketamine/propofol versus fentanyl/ propofol for sedating obese patients undergoing Endoscopic Retrograde Cholangiopancreatography (ERCP). Egyptian Journal of Anaesthesia 29: 207-211.
- Ma XX, Fang XM, Hou TN (2012) Comparison of the effectiveness of dexmedetomidine versus propofol target controlled infusion for sedation during coblation-assisted upper airway procedure. Chin Med J 125(5): 869-873.
- Dogan R, Erbek S, Gonencer HH, Erbek HS, Isbilen C, et al. (2010) Comparison of local anaesthesia with dexmedetomidine sedation and general anaesthesia during Septoplasty. Eur J Anaesthesiol 27(11): 960- 964.
- Tsai CJ, Chu KS, Chen TI, Lu DV, Wang HM, et al. (2010) comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during fibre optic nasotracheal intubation. Anaesthesia 65(3): 254-259.
- Darwish A, Sami R, Raafat M, Aref R, Hisham M (2012) Dexmedetomidine versus propofol for monitored anesthesia care in patients undergoing anterior segment ophthalmic surgery under peribulbar medial canthus anesthesia. Life Sci J 9(2): 789-793.
- Roback MG, Wathen JE, Bajaj L (2005) Adverse events associated with procedural sedation and analgesia in a pediatric emergency department: a comparison of parenteral drugs. Acad Emerg Med 12(6): 508-513.
- Blanchard AR (2002) Sedation and analgesia in intensive care. Medications attenuate stress response in critical illness. Postgrad Med 111: 59-60.
- Arain SR, Ebert TJ (2002) The efficacy side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intra operative sedation. Anesth Analg 95(2): 461-466.
- Muller S, Borowics SM, Fortis EA, Stefani LC, Soares G, et al. (2008) Clinical efficacy of dexmedetomidine alone is less than propofol for conscious sedation during ERCP. Gastrointest Endosc 67: 651-659.
- Prachanpanich N, Apinyachon W, Ittichaikulthol W, Moontripakdi O, Jitaree (2013) A comparison of dexmedetomidine and propofol in Patients undergoing electrophysiology study. J Med Assoc Нai 96(3): 307-311.
- Sudheesh K, Harsoor S (2011) Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth 55(4): 323-324.
- Grewal A (2011) Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol 27(3): 297-302.
- Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, et al. (2007) AGA Institute review of endoscopic sedation. Gastroenterology 133(2): 675-701.
- Mora-González JF, Robles Cervantes JA, Mora Martínez JM, Barba- Alvarez F, Llontop-3isfil Ede L, et al. (2012) Hemodynamic effects of dexmedetomidine fentanyl vs nalbuphine propofol in plastic surgery. Middle East J Anesthesiol 21(4): 553-537.
© 2018 Alaa Ali Elzohry. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






