- Submissions

Full Text
Aspects in Mining & Mineral Science
Metals from Ores: An Introduction
Fathi Habashi*
Department of Mining, Laval University, Canada
*Corresponding author: Fathi Habashi, Department of Mining, Metallurgical and Materials Engineering, Laval University, Quebec City, Canada
Submission: October 09, 2017; Published: December 11, 2017

ISSN: 2578-0255 Volume1 Issue1
Introduction
A mineral is a naturally occurring substance having a definite chemical composition, constant physical properties, and a characteristic crystalline form. Ores are a mixture of minerals: they are processed to yield an industrial mineral or treated chemically to yield a single or several metals. Ores that are generally processed for only a single metal are those of iron, aluminium, chromium, tin, mercury, manganese, tungsten, and some ores of copper. Gold ores may yield only gold, but silver is a common associate. Nickel ores are always associated with cobalt, while lead and zinc always occur together in ores. All other ores are complex yielding a number of metals.
Ores undergo a beneficiation process by physical methods before being treated by chemical methods to recover the metals. Beneficiation processes involve liberation of minerals by crushing and grinding then separation of the individual mineral by physical methods (gravity, magnetic, etc.) or physicochemical methods (flotation) Figure 1. Chemical methods involve hydrometallurgical, pyrometallurgical, and electrochemical methods. Metals and metalloids obtained from ores are shown in Figure 2.
Figure 1: Production of industrial minerals and metals from ores.

Classification of Minerals
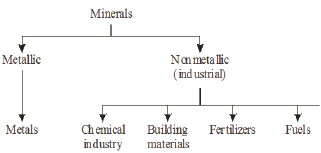
Minerals may be classified into two groups: metallic and non metallic. Metallic minerals are the chief raw materials for the manufacture of metals. Non metallic minerals which constitute about 75% of all the minerals, are so-called because they are not used for the manufacture of metals and also because of their lack of metallic lustre. Of these about 300 are used industrially in the chemical industry, in building materials, in fertilizers, as fuels, etc., and are known as the industrial minerals Figure 3.
Figure 2: Metals and metalloids obtained from ores.
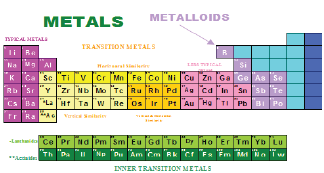
Figure 3: Classification of minerals.
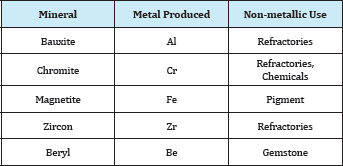
A metallic mineral may be used for the production of a metal, or after a minor treatment for the production of refractoriness or pigments. For example Table 1. Bauxite, the main source of aluminium: 90% is used in the manufacture of the metal and 10% in the manufacture of refractoriness, abrasives, and chemicals. Chromate, the main mineral for chromium, is used for manufacturing certain refractoriness as well as chemicals for the tanning industry. Magnetite is used for iron production and as a black pigment. Zircon, the main zirconium mineral is used for the production of specialized refractoriness. Beryl, the main beryllium mineral, when occurring in large transparent crystals, is a gemstone. When a mineral is used for more than on purpose, then its grade and the impurities present are the decisive factors in its utilization for metal production or otherwise. For example: Chromites ores [1]. These are classified into three grades: Metallurgical Ore with a high chromium content (minimum 68% chromium) and the chromium/iron ratio must not be less than 2.8/1, will be suitable for the manufacture of ferrochrome alloy or chromium metal.
Table 1: Metallic minerals for other uses than metal production.
Refractory Ore with a high aluminium oxide (the sum Cr2O3 and Al2O3 is more than 59%) would be suitable for the manufacture of refractoriness. Chemical Low-grade chromites, that are those with high iron content, are mainly used for the manufacture of dichromatic needed for the electroplating and tanning industry Table 2.
Table 2: Classification of chromite ores.
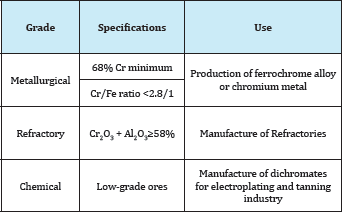
Table 3: Classification of manganese ores.
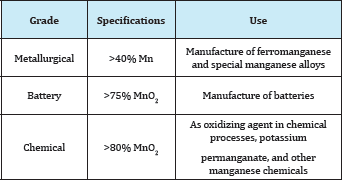
Manganese ore classified as follows Table 3. Metallurgical Ore with high manganese content (minimum 40% Mn) are suitable for the manufacture of ferromanganese and special manganese alloys.
An ore at least 75% MnO2 suitable for the manufacture of batteries.
An ore at least 80% MnO2 suitable for use as an oxidizing agent in chemical processes or in the production of potassium permanganate and other manganese chemicals.
Pyrite and pyrrhotite which are iron sulphides are usually considered as metallic minerals because of their metallic lustre but they are mainly evaluated for their sulphur and not for their iron content, they are used to make sulphuric acid. Few plants however, process the remaining ferric oxide to extract traces of nonferrous metals contained in them; the purified ferric oxide may then be used for making iron. The presence of pyrite and pyrrhotite in sulphide ores is undesirable and usually methods have to be found to remove them [2].
Ilmenite is a source of titanium as well as iron. Although titanium minerals are used for producing titanium metal, yet 99% of the tonnage is used for TiO2 pigment manufacture. Furthermore, ilmenite reserves are far larger than those of rutile; ilmenite supplies about 85% of the world demand and retile the remaining 15%.
While dolomite, (Mg,Ca)CO3, is used for producing metallic magnesium and to some extent as a refractory, magnetite, MgCO3, is used mainly as a refractory: hence it is classified as a non-metallic mineral. One reason for that is that MgO prepared from magnetite has a higher melting point than (Mg, Ca)O prepared from dolomite hence more suitable as a refractory.
Metallic
Table 4: Gives a list of the most important metallic minerals classified according to chemical composition.
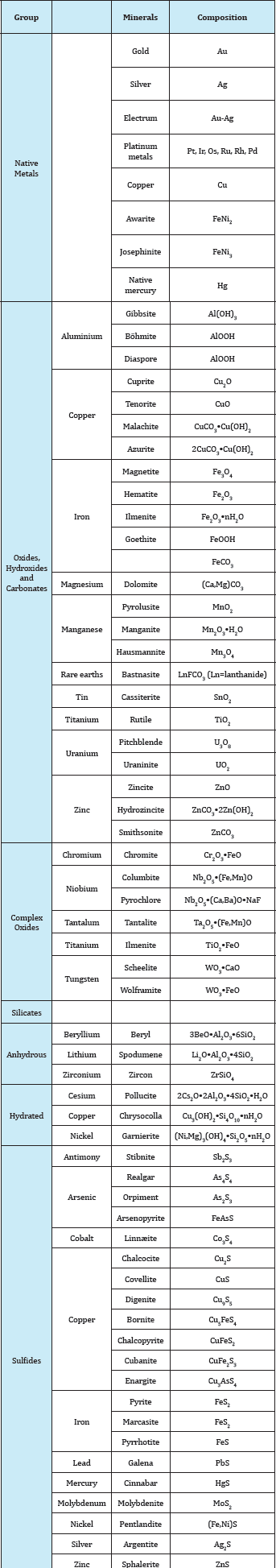
Table 4 Classification of the most important metallic minerals according to chemical composition of no technical importance Ln stands for lanthanide.
Native metals
Figure 4: Museum samples of native metals. Native mercury of no technical importance.

Table 5: Analysis of telluric and meteoric iron.
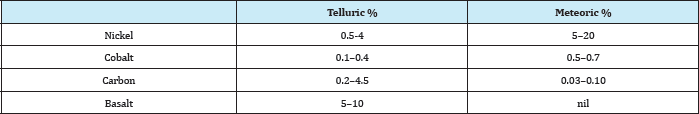
Figure 5: Museum samples of common oxide minerals.

Figure 4 shows museum samples of native metals. Iron also occurs in a rare form of large boulders 20 to 80 tonnes, which may be mistaken for a meteorite, but because of its different analysis Table 5 absence of Widmanstatten structure characteristic of meteoric iron when a piece is polished, etched, and examined by the optical microscope, it is known as telluric iron, i.e., terrestrial iron.
The major occurrence of telluric iron is in association with the basalts of Western Greenland. Large boulders are on exhibit at the Natural History Museums in Stockholm, Copenhagen, and Helsinki. Telluric iron is found also as small millimetre-sized peashaped grains disseminated in the basalt, characterized of their low carbon content, usually less than 0.7%. These were extracted from the basalt by the natives by crushing and then cold-hammering the collected metallic particles into coin-sized flakes to insert them into groves in bone and use them as knives [3].
Oxides, hydroxides, and carbonates
These comprise the important minerals of aluminium, iron, magnesium, manganese, rare earths, tin, titanium, and uranium; those of copper and zinc are of minor importance. Figure 5 shows some common oxide minerals. Complex oxides comprise minerals of chromium, niobium, tantalum, titanium, and tungsten.
Table 6: Formation of hydrated silicates.
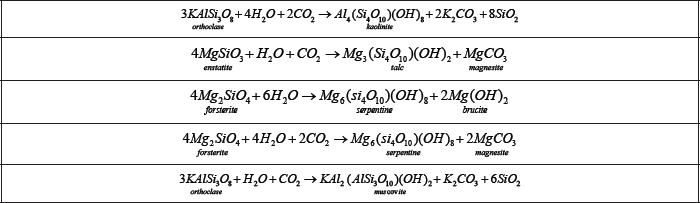
Silicates: These may be anhydrous and hydrated silicates Table 6. The first groupcomprise minerals of beryllium, lithium, and zirconium, while the second group comprise those of caesium, copper, and nickel.
Sulphides: These comprise the most important minerals of antimony, arsenic, cobalt, copper, lead, mercury, molybdenum, nickel, silver, and zinc. Figure 6 shows some common sulphide minerals.
Figure 6: Museum samples of common sulfide minerals.

Others: Phosphates are mainly the rare earths in the form of monazite sand. Sulphates of lead, gold telluride, and cobalt arsenide -all are of minor importance.
Non-metallic and industrial
According to their abundance, industrial minerals can be classified into three main groups: Rare: These occur in small quantities, in limited areas, used in small quantities, and command a high price. For example, diamonds, sheet mica, graphite, corundum, precious stones, and the semi-precious stones. Widely available: These occur in large quantities in few geologic environments, are used in appreciable amounts, and command a moderate price. For example asbestos, coal, phosphate, gypsum, kaolin, potash, salt, sulphur, talc, trine, barite, borates, feldspar, fluorite, magnetite, and diatomite.
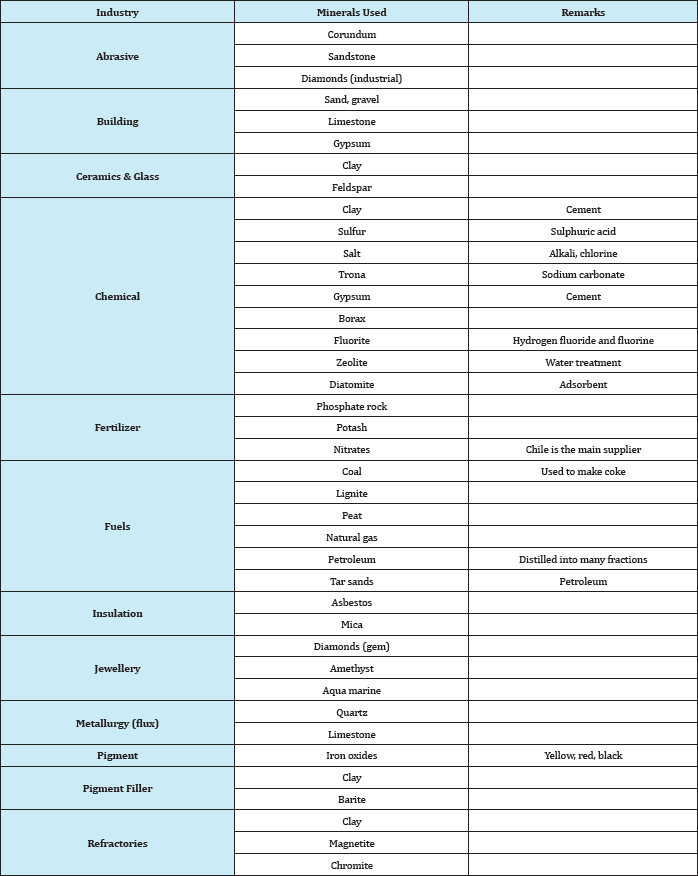
Abundant: These are abundant in all geologic environments, used in large amounts, and are relatively cheap. For example, clay, limestone, sand, gravel, and stones. Strictly speaking, some of the members of these groups are not minerals but ores having a geological name. For example, phosphate rock is neither a rock nor a mineral; it is a geological name for a certain type of formation containing phosphate minerals associated with gangue minerals such as calcite, iron oxides, clays, etc. The major phosphate mineral of economic value in this type of deposit is apatite, which is principally calcium phosphate. Similarly, clay is a geological name for a large variety of hydrated aluminium silicate minerals, of which kaolinite is one. Table 7 gives an alphabetical list of these minerals and their chemical composition. Industrial minerals can also be classified according to their use as shown in Table 8.
Table 7: Chemical composition of important industrial minerals.
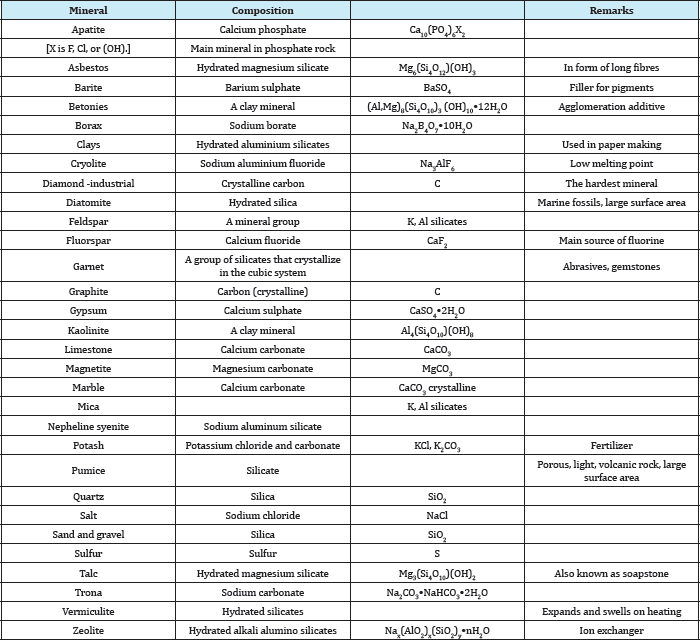
Table 8: Classification of industrial minerals according to their use.
Metals from the Sea
Magnesium is the only metal produced today from sea water. Sea water contains 0.13% magnesium from which magnesium hydroxide is precipitated and magnesium metal is produced. Dead Sea on the other hand contains a higher percentage of magnesium.
Summary
Metals used in daily life are produced by treatment of ores which contain minerals of these metals. Magnesium is the only metals obtained from sea water.
References
- Habashi F (2003) Metals from ores. An introduction to extractive.
- Habashi F (2016) Industrial minerals through the ages.
- Habashi F (2009) Metals from ores a look to the future. In: Krausz S (Ed.), In Proceedings of the XIII Balkan. Mineral Processing Congress, Romania, pp. 919-924.
© 2017 Fathi Habashi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






