- Submissions

Full Text
Advancements in Case Studies
Giant Glioblastoma in a Patient with Previous Prostate Adenocarcinoma
Anna Aldea Parés1, Adrián Téllez Santoyo1, Pedro Castro Rebollo2 and Ramón Estruch Riba3*
1Internal Medicine Service, Hospital Clínic de Barcelona, Spain
2Medical Intensive Care Unit, University of Barcelona, Spain
3Internal Medicine Service, Hospital Clínic de Barcelona, Spain
*Corresponding author: Ramón Estruch Riba, Internal Medicine Service, Hospital Clínic de Barcelona, Villarroel 170, Barcelona, Spain
Submission: April 19, 2018;Published: August 13, 2018

ISSN 2639-0531Volume1 Issue3
Abstract
Introduction: Malignant gliomas (GBM) are the most common primary malignant brain tumors. Clinical presentation is variable, being headache the most common symptom. Diagnosis is usually suspected by magnetic resonance (MRI) and in most of cases the treatment consists in neurosurgery followed by co-adjuvant radiotherapy and temozolomide.
Case presentation: A 57-year-old male presented to the Emergency Department with a 48-hour history of progressive holocraneal headache, vomiting, gait instability and bilateral hearing loss. He underwent brain Computed Tomography (CT) scan and MRI, with results compatible with GBM. Complete mass excision was performed without complications and he was discharged with co-adjuvant treatment with radiotherapy and temozolomide.
Conclusion: Despite optimal treatment and important advances in our understanding of molecular pathogenesis, GBM are still associated with high morbidity and mortality
Keywords: Glioblastoma; Temozolomide; Glioma; Brain tumor
Abbreviations:GBM: Glioblastoma; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; WHO: World Health Organization; IDH: Isocitrate dehydrogenase 1; MGMT: O6-methylguanine–DNA methyltransferase; EGFR: Epidermal Growth Factor Receptor; TKR: Tyrosine Kinase Receptor
Introduction
Malignant gliomas are invasive and histologically heterogeneous tumors derived from glia [1]. They account for 80% of malignant brain tumors, with an annual incidence of 5.26 per 100,000 population and 17,000 new cases diagnosed per year [2]. The incidence has increased over the past two decades, probably due to an improved diagnostic imaging [1]. It is more common in the sixth through eight decades of life [2], men [1] and Caucasian [1]. Their etiology remains unknown and the only established risk factor is exposure to ionizing radiation [1]. Gene polymorphisms that affect detoxification, DNA repair and cell-cycle regulation have been implicated in the development of gliomas [1]. Furthermore, approximately 5% of cases have a family history of gliomas and some of them are associated with rare genetic syndromes such as neurofibromatosis types 1 and 2, Li-Fraumeni syndrome and Turcot’s syndrome [1].
New-onset headache is the most frequent symptom, being present in about 50% of patients at diagnosis. It has a nonspecific pain pattern and progressive severity. Seizures are the presenting manifestation in about 20% to 40% of patients. Gait imbalance and incontinence may be present in mass effect tumors, and focal signs such as hemiparesis, sensory loss and visual field disturbances may reflect tumor location. Papilledema is less frequent due to early stage diagnosis [2]. CT scan is usually the first diagnostic test, and MRI with and without contrast is the one of choice and CT scan is reserved for patients unable to undergo it [2]. These imaging studies typically show a heterogeneously enhancing mass with surrounding edema and may have central areas of necrosis [1].
Case Presentation
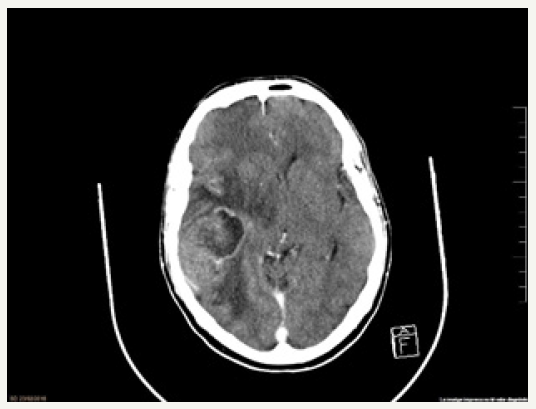
A 57-year-old male presented to the Emergency Department with complaints of stabbing holocraneal headache that didn’t cease with conventional analgesia, vomiting, gait instability and bilateral hearing loss over the last 48 hours. He had previous history of two colon polyps resected in 2012 with anathomopatological results of high and low-grade dysplasia; prostate adenocarcinoma (Gleason 9 T4N1M0) treated in 2015 with transurethral resection, radiotherap and double hormonal treatment; and liver hemangiomas. At presentation, his vital signs were normal. He was aware and oriented with a fluent speech. He had homonymous inferior quadrant-anopsia, central facial palsy and left-sided hemihypoesthesia with no other neurological impairment. He underwent a brain CT scan that showed a nodular lesion (46 x 42 x 42mm) in right temporal lobe with heterogeneous enhancement, extensive vasogenic edema, mass effect and a descending transtentorial herniation (Figure 1), suggestive of a single brain metastasis or a high-grade primary brain neoplasm. Intravenous dexamethasone was started, and he was transferred to an Intermediate Care Unit.
figure 1: Axial brain CT-scan with contrast enhanced lesion in right temporal lobe with extensive vasogenic edema and mass effect.
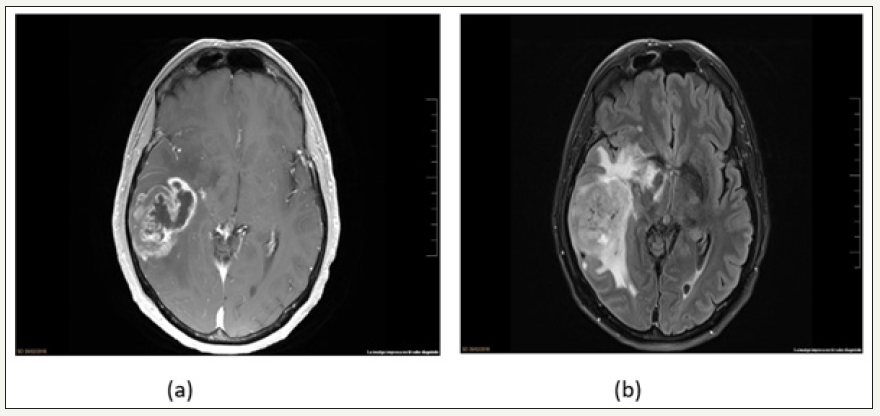
The brain MRI showed a right temporal neoplasm with areas of central necrosis, neo-angiogenesis and irregular contrast enhancement. The perfusion study showed an increment in the relative cerebral blood volume. The spectroscopic analysis demonstrated increment of the Cho peak, Cho/Cr ratio, Cho/NAA ratio and Lip/lac peak. All these findings were compatible with glioblastoma (GBM) (Figure 2). Complete mass excision under general anesthesia and neuro-navigation system was performed without complications. Post-operative course was uneventful. Anathomopatological examination demonstrated a GBM, grade IV of the World Health Organization (WHO) classification, wild-type Isocitrate dehydrogenase 1(IDH) and ATRX, nonspecific p53, and mild-immunoreactivity epidermal-growth factor receptor (EGFR) with methylated O6-methylguanine–DNA methyltransferase (MGMT). He was discharged, and he started concomitant adjuvant treatment with temozolomide and radiotherapy.
figure 2: Axial T1 with contrast and axial FLAIR brain MRI showed a right temporal expansive tumor, with central necrosis, angiogenesis, heterogeneous enhancement and vasogenic edema.
Discussion
After neuroimaging, patients with suspected malignant glioma should be considered for surgical resection that may lead to a rapid improvement of symptoms [2,3]. In 20-30% of patients, the disease is primarily unresectable and only a stereotactic diagnostic biopsy is performed [3]. Either surgical resection or biopsy should provide adequate tissue for histological and molecular characterization [2]. Based on histological features, the World Health Organization (WHO) classifies astrocytomas into four prognostic grades [1]: grade I (pilocytic astrocytoma), grade II (diffuse astrocytoma), grade III (anaplastic astrocytoma, oligodendroglioma and oligoastrocytoma) and grade IV (GBM). Grade III and IV are considered malignant gliomas. Anaplastic astrocytomas are characterized by increased cellularity, nuclear atypia and mitotic activity and GBM also contains areas of microvascular proliferation, necrosis or both [1].
Grade IV GBM accounts for approximately 50% of gliomas and is one of the most aggressive cancers [3]. It is usually described in two different clinical forms: primary GBM, which is the most common form (95%) and arises typically de novo within 3-6 months in older patients; and secondary GBM, which arises from prior low-grade astrocytomas over 10-15 years in younger patients [4]. In the past two decades, there has been extensive influx data describing GBM’s molecular pathogenesis as well as improved knowledge of cancer stem cells, which has given opportunities for newer therapies as well as promises for better control of the disease [4]. Three critical pathways are affected in approximately 80% of these brain tumors and include alterations of receptor tyrosine kinase/Ras/ phosphatidylinositol 3-kinase, p53 and retinoblastoma signaling [3]. Based on the growing understanding of molecular heterogeneity in GBM, the Cancer Genome Atlas divided this tumor in molecular subclasses: classical GBM (aberrant EGFR amplification, astrocytic cell expression pattern and loss of chromosome 10), mesenchymal GBM (NF1 and PTEN mutations and mesenchymal expression profile), proneural GBM (PDGFRA focal amplification, TP53 and IDH1 mutations with an oligodendrocytic cell expression) and neural GBM (normal brain tissue gene expression profile) [4-6].
Primary GBM has often amplified, mutated EGFR, which encodes altered EGFR, amplification of MDM2 gene, PTEN mutations and homozygous deletions of CDKN2A, whereas secondary GBM has increased signaling through PDGF-A receptor, p53 mutations, IDH1 mutations, MET amplification and overexpression of PDGFRA. Despite these molecular differences, both lead to improved activity and consequently to activation of RAS and PI3K pathways, playing a role in the increased cell proliferation, inhibition of apoptosis, invasion and angiogenesis [4-6]. Targeted molecular therapies have risen recently due to the better understanding of the molecular pathogenesis, especially inhibitors that target receptor tyrosine kinases such as EGFR, PDGFR and VEGFR as well as on signaltransduction inhibitors targeting mTOR and PI3K. However, the results have been disappointing as single agents had only response rates of 0 to 15% and no prolongation of 6-month progressionfree survival, probably because most malignant gliomas have coactivation of multiple tyrosine kinases and redundant signaling pathways [1].
A promising molecular GBM biomarker is O6-Methylguaninemethyl- transferase (MGMT) gene promoter metylation, which is present in 30-60% of GBM. MGMT is a repair protein that counteracts the effect of alkylating drugs like nitrosureas or temozolomide. Thus, epigenetic methylation of the MGMT gene promoter leads to repression of its expression and imply a favorable outcome in patients treated with alkylating agents [3]. The first-line adjuvant treatment after tumor resection surgery is radiotherapy (60Gy divided in 30 fractions) with chemotherapy consisting of the DNA alkylating agent temozolomide, which is administered orally [2- 4]. In a randomized phase III trial, on 562 patients, investigators demonstrated that the addition of temozolomide to short-course radiotherapy resulted in longer median overall survival (9.3 months) than short-course radiotherapy alone (7.6 months) [7]. This low survival can be explained by the persistence of residual tumor cells despite macroscopically complete resection due to its infiltrative growth pattern, leading to recurrence of tumor in most of the patients [3]. The most important adverse prognostic factors are advanced age, poor Karnofsky performance status and unresectable tumor [1]. Despite aggressive multimodal therapy, most GBM patients eventually die from their disease, presenting severe symptoms in the terminal disease phase such as seizures, headache, drowsiness, dysphagia, agitation and delirium, that need appropriate palliative care [3].
References
- Wen P, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359 (5): 492-507.
- Omuro A, DeAngelis (2013) Glioblastoma and other malignant gliomas. JAMA 310(17): 1842-1850.
- Preusser M, Ribaupierre S, Wöhrer A, Erridge S, Hegi M, et al. (2011) Current concepts and management of glioblastoma. Ann Neurol 70 (1): 9-21.
- Alifieris C, Trafalis D (2015) Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol Ther 152: 63-82.
- Bastien J, McNeill K, Fine H (2014) Molecular characterizations of glioblastoma, targeted therapy and clinical results to date. Cancer 121(4): 502-516.
- Cloughesy T, Cavenee W, Mischel P (2014) Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol 9: 1-25
- Perry J, Lapierre N, O’Callaghan C, Brandes A, Menten J, et al. (2017) Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med 376: 1027-1037.
© 2018 Ramón Estruch Riba. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






