- Submissions

Full Text
Advances in Complementary &Alternative Medicine
Preliminary phytochemical analysis and in vivo evaluation of antipyretic effects of methanolicextract of Argyreia pilosa Wight & Arn.
DSNBK Prasanth1*, Atla Srinivasa Rao2 and Rajendra Prasad Yejella3
1Department of Pharmacy, JNTUK, India
2Department of Pharmaceutical Analysis and Quality Control, Shri Vishnu College of Pharmacy, India
3Department of Pharmaceutical Chemistry, University College of Pharmaceutical Sciences, India
*Corresponding author: DSNBK Prasanth, Research Scholar, Department of Pharmacy, JNTUK, Kakinada-533 003, Andhra Pradesh, India
Submission: December 15, 2017; Published: February 08, 2018

ISSN: 2637-7802Volume1 Issue2
Abstract
The antipyrexia action of the methanol extract of A. pilosa had been explored utilizing the yeast evoked pyrexia procedure in rabbits. Paracetamol utilized as a positive control as well as negative control group acquired distilled water. Rectal temperatures of all rabbits had been documented instantly prior to the administration of the extract or vehicle or paracetamol as well as again at 30min period for 3h utilizing digital thermometer. The extract had been additionally phytochemically tested with regard to alkaloids, tannins, saponins, flavonoids, cardiac glycosides and phenols. At 400mg/kg dosage the extract revealed considerable decrease in yeast evoked raised temperature when compared with that of standard drug paracetamol where by the extract dose 200mg/kg had been less effective as compared to higher dose (p<0.05). Phytochemical testing demonstrated the existence of flavonoids, alkaloids, tannins, phenols, steroids, acid compounds, glycosides, amino acids, and proteins. This research confirmed that this methanol extract of A. pilosa at a dose of 400mg/kg owns considerable antipyretic outcome against the yeast-induced raised temperature. The antipyretic activity of A. pilosa extract could be due to its secondary metabolites, which probably consist of flavonoids like Rutin and Quercetin; sterols like β-Sitosterol. But, further phytochemical, as well as biological tests, are recommended to determine the other active chemical constituents accountable for the antipyretic activity.
Keywords: Argyreia pilosa; β-Sitosterol; Phytochemical analysis; Antipyretic; Rutin
Introduction
The bondage among human health and plants can be found from fossils history regarding 60,000 years back [1]. About 215,000 to 500,000 species of higher plants exist on our planet. However, merely 6% of plants are utilized for the pharmacological activity Nearly 122 compounds have been isolated from 94 species of plants as well as 80% of these compounds have been employed for the same intention or relevant purpose [2]. Pyrexia or fever is described as the elevation of body temperature. It is a natural defensive system or responds to damaged tissues, inflammation, malignancy, or graft rejection. As a result of inadequate personal hygiene practices and malnutrition, children from developing nations endure numerous infections that present as a fever. These kinds of fevers are usually associated with personal discomforts for example pain (myalgia) that definitely results in morbidity along with fatality [3].
In the present investigation, we are focusing our exploration on one of the commonly available plants in India i.e., Argyreia pilosa (A. pilosa), belongs to family Convolvulaceae. The family Convolvulaceae includes almost 1650 mainly exotic species. The genus Argyreia, with near 135 species, a few of the significant species such as A.aggregate, A. cuneata, A. cymosa, A. daltoni, A. elliptica, A. fulgens, A. kleiniana, A. malabarica, A. nervosa, A. pilosa, A. setosa, A. strigosa and A. speciosa [4-6]. Every part of this plant tends to be widely used as a folklore medicine for the remedying of several health conditions by the Indian traditional healer. Traditionally, the leaves paste is placed on the neck area for a cough, quinsy and used externally in case of itch, eczema along with other skin troubles, antidiabetic and antiphlogistic [6].
A. pilosa is a Twiner, branchlets reddish and hirsute; leaves simple, alternate, broadly ovate, 7-10*7-9cm, apex acute, base subcordate, margin entire, and nerves prominent up to 7-8 pairs. Flowers pink, in axillary, capitates heads, peduncle long 2-3cm, bracts linear, bristly hair to 1cm long, calyx 5 lobed, lobes unequal, nearly free to base, oblong-lanceolate to 0.8cm long, corolla infundibular, to 4cm, lobes spreading, stamens included Fruit berry [7,8]. A vast range of phytochemical constituents has been separated from the genus Argyreia i.e., glycosides, alkaloids, amino acids, proteins, flavonoids, triterpene and steroids [9].
Ethnomedicinally, the genus Argyreia has been documented various pharmacological activities including nootropic, aphrodisiac, antioxidant, antiulcer, immunomodulatory, hepatoprotective, anti-inflammatory, antihyperglycaemic, antidiarrheal, antimicrobial, antiviral, nematicidal, anticonvulsant, analgesic, anti-inflammatory, wound healing and central nervous depressant activities [9-13]. Therefore, existing had been carried out to assess the antipyretic potential of methanolic extract of A. pilosa against baker's yeast- induced fever in rabbits as the drugs having anti-inflammatory activity [14] can show antipyretic activity too.
Materials and Methods
Animal used
Local strain, healthy male and female adult rabbits (1-1.5kg) were used for the study and kept in the departmental animal house, VV Institute of Pharmaceutical Sciences, Gudlavalleru. Before the study, rabbits had been acclimatized in a controlled temperature of 22-25 °C and light/dark cycles for 12/12h for one week. Animals were maintained on the standard diet and water ad libitum. These fasted overnight prior to study nevertheless water was handed in free accessibility. The experiment was carried out according to guidelines "Committee for the Purpose of Control and Supervision" (CPCSEA). (Approval No:
1847/PO/Re/S/16/CPCSEA)
Drugs, reagents, and apparatus used
Paracetamol (GlaxoSmithKline), Baker yeast (Loba Chem, Mumbai), Distilled water, Digital Thermometer (Medisign MANA).
Plant Material
The plant material was purchased from Tirupati, Chittoor region of Andhra Pradesh, India throughout the month of March 2016 and identified by Dr. K. Madhava chetty, Taxonomist, Sri Venkateswara University Tirupati, India. Voucher specimen No. 1922 was placed at the herbarium of VV Institute of Pharmaceutical Sciences, Gudlavalleru for future reference.
Preparation of extract
The freshly gathered plant material was shade dried and pulverized. The powder (1Kg) was treated with petroleum ether for the removal of fatty and waxy material. After that, it had been air dried and macerated with methanol, strained and concentrated at 45 °C in Buchi rotavapor. The weight of methanolic extract obtained was 75g (7.5% w/w yield). The methanolic extract had been revoked in distilled water in a separating funnel and partitioned sequentially with petroleum ether, chloroform, ethyl acetate and n-butanol to acquire fractions in these solvents. Eventually, left residual aqueous fraction at the end was collected. The solvents were removed on a rotary evaporator at low pressure to obtain dried fractions. These extracts were subjected to preliminary phytochemical screening and these extracts were stored in the refrigerator at 4 °C for further use [15].
Phytochemical screening
The various extract of A. pilosa was subjected to qualitative chemical analysis by using standard procedures as follows.
The phytochemical screening of carbohydrates was detected by molisch's test; Proteins were detected by using two tests namely Biuret test and millon's test and amino acids by Ninhyrdin's test; Steroids was detected by salkowski, Libermann-Burchards and Libermann's test; Alkaloids was identified with freshly prepared Dragendroff's Mayer's, Hager’s and Wagner's reagents and observed for the presence of turbidity or precipitation. The flavonoids were detected using four tests namely Shinoda, sulfuric acid, aluminum chloride, lead acetate, and sodium hydroxides. Tannins were detected with four tests namely gelatin, lead acetate, potassium dichromate and ferric chloride. The froth, emulsion, and lead acetate tests were applied for the detection of saponins. The steroids were detected by (acetic anhydride with sulfuric acid) and (acetic chloride with sulfuric acid) tests. Sample extracted with chloroform was treated with sulfuric acid to test for the presence of terpenoids. Ammonia solution and ferric chloride solutions were used to the presence of anthraquinone [16-19].
Isolation of constituents
Petroleum ether extract (PEE) was subjected to silica-gel (100-200mesh) column (length 100cm and diameter 3cm) chromatography (elution rate of 2ml min-1 flow with a total elution of 200ml) and eluted with Petroleum ether and ethyl acetate in different proportions. The consequent fractions (Fr) were collected and spotted over pre-coated silica gel F254 plates (20x20cm, Merck, Germany). The optimum resolution was achieved with chloroform and ethyl acetate (5:5v/v) solvent system and the plates were sprayed with anisaldehyde-sulphuric acid reagent to visualize the spots. The chloroform fraction was subjected to chromatography on silica gel (60-120mesh, Merck) eluted with chloroform: ethyl acetate (5:5) solvent system. Repeated chromatography to give major two steroids ie., PC-1 (β-Sitosterol) and PC-2 (Stigmasterol) (Rajput & Rajput, 2012). After extraction, the aqueous layer was collected and left to stand in a cold place for 72 hours; a yellow precipitate separated out of the solution. The precipitate was filtered and washed with a mixture of chloroform: ethyl acetate: ethanol (50:25:25). The un-dissolved part of the precipitate was dissolved in hot methanol and filtered, the filtrate was evaporated to dryness to give 115mg yellow powder i.e, PC-3 (rutin), and its melting point was measured [13]. The Ethyl acetate fraction was chromatographed using the flash column on a Silica gel eluted with chloroform-methanol step-gradient (starting with 100:0 to 4:1), eluted fractions were combined on their TLC pattern to yield 8 fractions. The chloroform-methanol fraction (10:1) was chromatographed on a Sephadex LH-20 column eluted with chloroform-methanol (1:1) to yield PC-4 (Quercetin) [20].
Antipyretic activity
The rabbits had been arbitrarily divided into four groups each comprising of six animals (n=6). Each of the groups was initially administered with baker yeast (Saccharomyces cerevisiae) (3mL/ kg of 10% suspension subcutaneous) for induction of fever [21,22]. Immediately after 4h of yeast administration, Group I animals were given with distilled water and served as negative control. Group II animals were treated with paracetamol by oral administration and considered as positive control. Group III and IV animals were treated with A. pilosa methanolic extract 200 and 400mg/kg body weight respectively. The dose of A. pilosa was selected by an effective dose fixation study method with slight modification [23]. The rectal temperature had been assessed using the digital thermometer (Medisign) layered with glycerin (as a lubricant). After baker yeast injection, the rectal temperature had been documented half and hourly. The animals exhibited rises in temperature of 0.5-1 °C during 4th hr were included in the study. After 4h of yeast injection, each of the screened samples had been given orally by using the syringe. After drug administration, rectal temperature was recorded half an hourly for 3h.
Statistical analysis
Statistical analysis was carried out using Graph Pad Prism 5.0 (Graph Pad Software, San Diego, CA). All results were expressed as mean±SD. The data were analyzed by one-way ANOVA followed by Tukey multiple comparison tests.
Result
Phytochemical screening
Table 1: Preliminary phytochemical screening of various extracts of Argyreia pilosa.
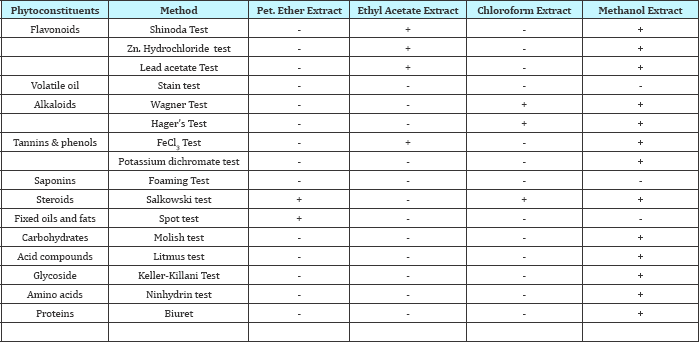
+ Present; - Absent
The phytochemical screening for various extracts viz., petroleum ether, chloroform, ethyl acetate, and methanol were carried out and results were displayed in Table 1.
Characterization of isolated Phytoconstituents
Stigmasterol: White powder, C29H48O, MW 412.69. UV λmax (CHCl3)nm: 257; IR (KBr) vmax 3418 (-OH), 2934, 2866, 2339, 1602, 1566, 1461, 1409, 1383, 1251, 1191, 1154, 1109, 1089, 1053, 1020, 791 cm-1; ESMSm/z (%): 409.2, 395.3, 335, 161, 144, 121.1, 105.1, 97.1, 85.1, 69, 67.2, 65, 50.2; 1H NMR (400 MHz,CDCl3) δppm: 7.25 (1H,s,OH-2), 5.34-5.35 (1H,d), 5.12-5.18 (1H,m), 4.995.05 (1H,m), 3.48-3.56 (1H,m), 2.18-2.31 (2H,m), 1.93-2.09 (3H,m), 1.82-1.87 (2H,m), 1.66-1.75 (1H,m), 1.37-1.54 (13H,m), 1.05-1.31 (m,7H), 0.99-1.01 (m,8H), 0.90-0.98 (m,2H), 0.78-0.85 (m,9H), 0.66-0.70 (3H,t); 13C NMR (400 MHz,CDCl3) δppm: 140.85 (C-4), 138.31 (C-19), 129.40 (C-20), 121.72 (C-7), 77.34 (C-2), 71.86 (C- 11), 56.95 (C-17), 56.09 (C-21), 51.29 (C-10), 50.29 (C-12), 42.41 (C-3), 42.30 (C-18), 40.46 (C-13), 39.77 (C-5), 37.35 (C-6), 36.59 (C- 8), 32 (C-9), 31.96 (C-1), 31.91 (C-22), 31.77 (C-16), 28.91 (C-15), 25.41 (C-24), 24.41 (C-23), 21.24 (C-26), 21.14 (C-14), 21.06 (C- 29), 19.42 (C-27), 19.03 (C-25), 12.23 (C-28). PC-01 was identified as Stigmasterol.
β-Sitosterol: White powder, C29H500, MW 414.70; UV λmax(CHCl3) nm: 251; IR (KBr) vmax 3424, 2959, 2936, 2867, 1602, 1565, 1465, 1382, 1332, 1242, 1191, 1154, 1051, 779, 450, 432, 416cm- 1; ESMSm/z (%): 411.2, 397.3, 383.3, 311.2, 161.1, 81.2; 1H NMR (400 MHz,CDCl3) δppm: 7.30 (1H,s), 5.34-5.35 (1H, d), 4.98-5.19 (1H, m), 3.47-3.55 (1H,m), 2.19-2.31 (2H,m), 1.03-1.30 (9H,m), 1.00 (4H,s), 0.90-0.98 (4H,m), 0.76-0.86 (9H,m), 0.68-0.69 (3H,d), 1.94-2.07 (2H,m), 1.79-1.88 (4H,m); 13C NMR (400MHz, CDCl3) δppm: 140.84 (C-4), 121.70 (C-7), 71.82 (C-2), 56.94 (C-11), 56.85 (C-17), 50.25 (C-10), 45.95 (C-21), 42.39 (C-7), 42.36 (C-3), 39.87 (C-13), 37.34 (C-5), 36.57 (C-6), 36.19 (C-18), 33.78(C-19), 32.15 (C-8), 31.99 (C-9), 31.97 (C-7), 30.39 (C-22), 26.28 (C-20), 25.90 (C-15), 25.40 (C-16), 24.40 (C-24), 23.2 (C-23), 21.17 (C-26), 21.06 (C-14) 21.06 (C-29), 19.32 (C-27), 19.34 (C-25), 12.11 (C-28). PC- 02 was identified as β-Sitosterol.
Rutin: Yellow powder, C27H30O16, MW 610.52 ; UV λmax(EtOH) nm: 203; IR (KBr) vmax 1001, 1013, 1065, 1092, 1150, 1166, 1203, 1295, 1362, 1458, 1504, 1566, 1601, 1649, 2340, 3422cm-1; ESMSm/z (%): 609.1 (M-1)-, 610, 301; 1H NMR (400MHz, DMSO) δppm: 12.6 (1H,s), 10.84 (1H,s), 9.68 (1H,s), 9.18 (1H,s), 7.557.56 (1H,d), 7.54 (1H,s) 6.84-6.86 (1H,d), 6.39 (1H,d), 6.2 (1H,d), 5.34-5.36 (1H,t), 5.29 (1H,d), 5.11 (1H,s), 5.07-5.09 (1H,d), 4.53 (1H,s), 4.39 (2H,s). 4.35 (1H,s), 3.70-3.72 (1H,d), 3.21-3.32 (1H,m), 3.05-3.10 (2H,t); 13C NMR (400MHz, DMSO) δppm: 177.35 (C-4), 164.03 (C-7), 161.20 (C-5), 156.57 (C-8a), 156.40 (C-2), 148.37 (C- 4'), 144.71 (C-5'), 133.31 (C-3), 121.56 (C-1'), 121.18 (C-2'), 116.26 (C-3'), 115.21 (C-6'), 103.96 (C-6'"), 101.19 (C-6"), 100.70 (C-4a), 98.65 (C-6), 93.55 (C-8), 76.46 (C-2''), 75.90 (C-4''), 74.06 (C-5''), 71.85 (C-2’"), 70.56 (C-5’"), 70.35 (C-3’’’), 70.01 (C-4’’’), 68.20 (C- 3”), 66.97 (C-2a), 17.68 (C-2’”). PC-03 was identified as Rutin.
Quercetin: Yellow powder, C15H1007, MW 302.23; UV λmax(EtOH) nm: 210; IR (KBr) λmax 3413, 2340, 1607, 1565, 1523, 1462, 1408, 1383, 1320, 1263, 1199, 1168, 1131, 1014, 959, 782, 465, 457, 441, 423cm-1; ESMSm/z (%): 301 (M-H). 301.9, 300; 1H NMR (400MHz, DMSO) δppm:) 12.49 (1H,s), 10.77 (1H s). 9.57 (1H,s), 9.29-9.33 (2H,d), 7.68-7.69 (1H,d), 7.53-7.69 (1H,m), 6.88-6.90 (1H,d), 6.41 (1H,d), 6.19 (1H,d); 13C NMR (400MHz) 175.81 (C- 7), 163.85 (C-1), 160.70 (C-3), 156.17 (C-5), 147.67 (C-9), 146.81 (C-15), 145.03 (C-8), 135.68 (C-11), 121.96 (C-12), 119.96 (C-13), 115.59 (C-16), 115.08 (C-4), 103.01 (C-2), 98.16 (C-6), 93.33. PC-04 was identified as Quercetin.
Antipyretic Activity
Figure 1: Line graph showing the effect of paracetamol and methanolic extract of Argyreia pilosa on yeast-induced pyrexia.
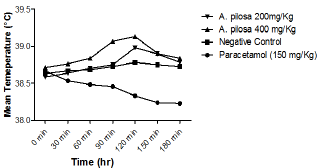
Figure 2: Radar diagram showing the effect of the Methanolic extract of Argyreia pilosa (MAR) and paracetamol on yeast-induced pyrexia model.
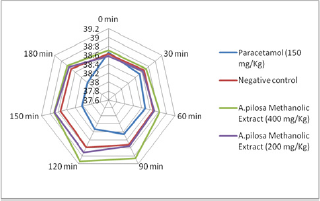
Subcutaneous injection of the pyrogenic dose of yeast produced elevated changes in rectal temperature, which is shown in Table 1 and showed that maximum temperature in negative control group was attained during 120min of S. cerevisiae administration. The administration of methanolic extract of A. pilosa (200mg/kg and 400mg/kg) and paracetamol reduced the rectal temperature significantly. The effects of extract at a dosage of400 and 200mg/kg reduced the temperature but the effect was slow and less significant as compared to Paracetamol. The MAP showed a significant (P<0.001) decrease in rectal temperature at doses 200 and 400mg/ kg when compared with the standard. Extract at doses of 200 and 400mg/kg showed a progressive decline in mean temperature pattern with the increase in the dose. Paracetamol showed significant (P<0.001) decrease in rectal temperature. The onset of action of paracetamol was 30min. MAP at doses 200 and 400mg/ kg showed its onset of action from 150min (Figure 1). The line graph (Figure 1) shows that paracetamol registered a phenomenal decrease in mean temperature from 38.66±0.21 to 38.22±0.4. Figure 1 illustrates that the most significant (P<0.05) decrease in mean temperature in this study was shown by paracetamol followed by MAP at 400mg/kg. Statistical analysis was performed using one-way analysis of variance followed by Tukey's test. The consequences of extract at a dose of 400mg/kg were nearly much like that of standard drug paracetamol. The results were recorded as mean±SD. Out of all the groups apart from negative control, temperature became normal through 3 h of study (Figure 1 & 2 and Table 2).
Table 2: Effect of methanolic extract of Argyreia pilosa on yeast extract induced pyrexia in rabbits.
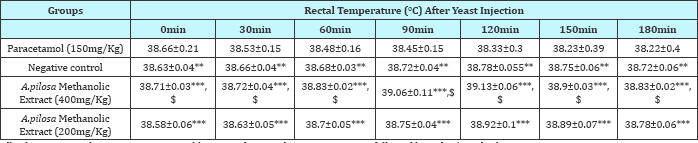
All values expressed as mean±SEM; n=3 rabbits in each group, by one-way ANOVA followed by Tukey’s Multiple Comparison Test.
***: p<0.001 Vs Paracetamol (150mg/Kg); **: p<0.01 Vs Paracetamol (150mg/Kg) and $: p<0.05 Vs Control
Discussion
Fever is the main defensive reaction referred to as the "acute phase reaction", which happen throughout inflamed processes of various sources. Brewer's yeast (lipopolysaccharide that is the cell wall element of Gram-negative bacteria) is an exogenous pyrogen which binds to the immunological protein referred to as lipopolysaccharide binding protein. This kind of binding leads to the synthesis as well as the release of numerous endogenous cytokine factors, for example, interleukin (IL)-1, IL-6, and TNFα, that trigger the arachidonic acid pathway, and also eventually result in the synthesis and release of prostaglandin E2 (PGE2). Yeast-induced pyrexia is termed as pathogenic fever [3].
Based on the traditional perspective, fever is evoked through inflamed mediators (I L-1, IL-2, TNFα, others) unveiled through the peripheral mononuclear macrophages and other immune cells [24,25]. These fever-promoting cytokines tend to be moved through the bloodstream to the brain by particular carriers [26]. Cytokines are carried through the blood stream and also enter the brain through the circumventricular organs [27]. On the other hand, the cytokines might interact with their receptors on brain endothelial cells or perivascular tissue [28]. This presumed mechanism of fever induction is called the humoral hypothesis of fever induction. These pro-inflammatory mediators address the preoptic/anterior hypothalamus activating the release of PGE2 made out of cyclooxygenase (COX)-2 and therefore increasing the body temperature [29].
An efficient antipyretic like paracetamol serves through embarrassing the effect of these pyrogens in the temperaturesensitive neurons in the preoptic region of the hypothalamus to COX formation of PGE2 [30]. An efficient antipyretic like paracetamol serves through embarrassing the effect of these pyrogens in the temperature-sensitive neurons in the preoptic region of the hypothalamus to COX formation of PGE2.
In this study, orally administered paracetamol at 150mg/kg significantly attenuated baker's yeast-induced fever in rabbits. Our study results are matching to other studies that have also shown the reduction of temperature in rabbits by paracetamol at the same dose [31]. Antipyretics and non-steroidal anti-inflammatory drugs (NSAIDS) reduce temperature by inflammation reduction in the peripheral and CNS thermoregulatory sites [32]. In the current study, A. pilosa methanolic extract reduced baker’s yeast-induced fever in rabbits significantly. The preliminary phytochemical study indicated that extract contains flavonoids, alkaloids, tannins, phenols, steroids, fixed oils, carbohydrates, glycosides, amino acids, and proteins. The presence of these bioactive compounds may be responsible for the antipyretic activity of this extract as flavonoids like quercetin and rutin [33] and sterols like β-Sitosterol [34] have the antipyretic effect.
However, the previous studies on Argyreia nervosa documented that methanolic and ethyl acetate extract revealed considerable action towards yeast evoked fever because of inhibition of prostaglandins synthesis may be the feasible mechanism [35].With this research, that they did not investigate the probable constituents accountable for antipyretic action. However, our exploration exposed the phytoconstituents ie, flavonoids like quercetin and rutin [33] and sterols like ß-Sitosterol [34] which are liable for antipyretic activity.
The genus Argyreia has various types of flavonoids such as quercetin, kaempferol, kaempferol 3-O-L-rhamnopyranoside, 7,8,3',4',5’-pentahydroxyflavone, 5-O-α-L-rhamnopyranoside and 7,8,3',4',5’-pentahydroxyflavone 5-O-β-D-glucopyranoside [9]. A study reported the inhibitory effects of kaempferol and quercetin against fever causing inflammatory mediators [36]. It could be assumed that A. pilosa have antipyretic effect by reducing the concentration of PGE2 in the hypothalamus or by interrupting the steps that connect the peripheral inflammation with the central production of PGE2 or both [37,38].
Conclusion
It is concluded that methanolic extract of A. pilosa has significant antipyretic activity. This property was attributed to the presence of Rutin, β-Sitosterol, and Quercetin in the MAP So, the traditional use of A. pilosa in fever is supported by this study and it would encourage its use in fever with a greater degree of assurance of its efficacy. It is recommended to determine the other active chemical constituents accountable for the antipyretic activity.
References
- Fabricant DS, Farnsworth NR (2001) The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109(Suppl 1): 69-75.
- Farnsworth J (1988) Screening plants for new medicines. Biodiversity. In: Wilson EO (Ed.), National Academy Press, Washington DC, USA.
- Bhattacharya A, Behera R, Agrawal D, Sahu PK, Kumar S, et al. (2014) Antipyretic effect of ethanolic extract of moringa oleifera leaves on albino rats. Tanta Medical Journal 42(2): 74-78.
- Traiperm P, Staples GW (2014) A new endemic thai species of Argyreia (Convolvulaceae). Phytotaxa 164(4): 281-285.
- Chetty KM, Sivaji K, Rao KT (2008) Flowering plants of chittoor district, Andhra Pradesh, India. Student Offset Printers, Tirupati, India.
- Ambasta S (1986) The useful plants of India. CSIR, India. pp. 918.
- Manjunatha B (2004) Flora of davanagere district, Daya Books, Karnataka, India.
- Matthew KM (1995) An excursion flora of central Tamilnadu, CRC Press, India.
- Galani V, Patel B, Patel N (2010) Argyreia speciosa (Linn. f.) sweet: A comprehensive review. Pharmacogn Rev 4(8): 172-178.
- Lalan BK, Hiray R, Ghongane B (2015) Evaluation of analgesic and antiinflammatory activity of extract of holoptelea integrifolia and argyreia Speciosa in animal models. J Clin Diagn Res 9(7): FF01-FF04.
- Yadav KS, Yadav NP, Rawat B, Rai VK, Shanker K, et al. (2014) An assessment of wound healing potential of Argyreia speciosa leaves. The Scientific World Journal 2014.
- Galani VJ, Patel BG (2011) Psychotropic activity of argyreia speciosa roots in experimental animals. Ayu 32(3): 380-384.
- Motawi TK, Hamed MA, Hashem RM, Shabana MH, Ahmed YR (2012) Protective and therapeutic effects of argyreia speciosa against ethanol- induced gastric ulcer in rats. Z Naturforsch C 67(1-2): 47-57.
- DSNBK P, Rao A, Prasad Y (2017) Analgesic and anti-inflammatory properties of Argyreia Pilosa Wight & Arn Open Access Journal of Pharmaceutical Research 1(4): 1-12.
- Ahmed D, Saeed R, Shakeel N, Fatima K, Arshad A (2015) Antimicrobial activities of methanolic extract of Carissa opaca roots and its fractions and compounds isolated from the most active ethyl acetate fraction. Asian Pac J Trop Biomed 5(7): 541-545.
- Alam F, Najum us Saqib Q (2015) Pharmacognostic standardization and preliminary phytochemical studies of gaultheria trichophylla. Pharm Biol 53(12): 1711-1718.
- Harborne JB (1973) Phytochemical methods; a guide to modern techniques of plant analysis. Chapman & Hall, London.
- Khandelwal KR (2008) Practical pharmacognosy : techniques and experiments. Niral Prakashan, Maharashtra, India.
- Raaman N (2006) Phytochemical techniques. New India Publ Agency, Pitam Pura, New Delhi, India.
- Wan C, Yu Y, Zhou S, Tian S, Cao S (2011) Isolation and identification of phenolic compounds from Gynura divaricata leaves. Pharmacogn Mag 7(26): 101-108.
- Hossain E, Mandal SC, Gupta J (2011) Phytochemical screening and in-vivo antipyretic activity of the methanol leaf-extract of bombax malabaricum DC (Bombacaceae). Tropical Journal of Pharmaceutical Research 10(1): 55-60.
- Sultana S, Akhtar N, Asif HM (2013) Phytochemical screening and antipyretic effects of hydro-methanol extract of melia azedarach leaves in rabbits. Bangladesh Journal of Pharmacology 8(2): 214-217.
- Pandikumar P, Babu NP, Ignacimuthu S (2009) Hypoglycemic and antihyperglycemic effect of begonia malabarica Lam in normal and strep tozotocin induced diabetic rats. J Ethnopharmacol 124(1): 111115.
- 24. Zeisberger E (1999) From humoral fever to neuroimmunological control of fever. J Therm Biol 24(5): 287-326.
- Roth J (2006) Endogenous antipyretics. Clin Chim Acta 371(1-2): 13-24.
- Banks WA, Plotkin SR, Kastin AJ (1995) Permeability of the blood-brain barrier to soluble cytokine receptors. Neuroimmunomodulation 2(3): 161-165.
- Roth J, Harre EM, Rummel C, Gerstberger R, Hubschle T (2004) Signaling the brain in systemic inflammation: role of sensory circumventricular organs. Front Biosci 9(7): 290-300.
- Schiltz JC, Sawchenko PE (2003) Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci 8: s1321-s1329.
- Saper CB, Breder CD (1994) The neurologic basis of fever. N Engl J Med 330(26): 1880-1886.
- Ashok B, Ravishankar B, Prajapati P, Bhat SD (2010) Antipyretic activity of guduchi ghrita formulations in albino rats. Ayu 31(3): 367-370.
- Ahmad S, Rehman T, Abbasi WM (2017) Effects of homoeopathic ultrahigh dilutions of aconitum napellus on baker's yeast-induced fever in rabbits. Journal of Integrative Medicine 15(3): 209-213.
- Jongchanapong A, Singharachai C, Palanuvej C, Ruangrungsi N, Towiwat P (2010) Antipyretic and antinociceptive effects of Ben-cha-Lo-Ka-Wi- Chian remedy. Journal of Health Research 24(1): 15-22.
- Eldahshan OA, Abdel-Daim MM (2015) Phytochemical study, cytotoxic, analgesic, antipyretic and anti-inflammatory activities of Strychnos nux- vomica. Cytotechnology 67(5): 831-844.
- 34. Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K, et al. (1980) Antiinflammatory and antipyretic activities of beta-sitosterol. Planta Med 39(2): 157-163.
- Jeet K, Tomar S, Thakur N (2012) Antipyretic activity of whole aerial part from Argyreia nervosa. Int J Pharm Pharm Sci 4(4): 76-77.
- Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E (2007) Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007.
- Li S, Dou W, Tang Y, Goorha S, Ballou LR, Blatteis CM (2008) Acetaminophen: antipyretic or hypothermic in mice? In either case, PGHS-1b (COX-3) is irrelevant. Prostaglandins Other Lipid Mediat 85(3): 89-99.
- Park EJ, Cheenpracha S, Chang LC, Kondratyuk TP, Pezzuto JM (2011) Inhibition of lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression by 4-[(2'-O-acetyl-alpha- L-rhamnosyloxy)benzyl] isothiocyanate from Moringa oleifera. Nutr Cancer 63(6): 971-982.
© 2018 DSNBK Prasanth, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






