- Submissions

Full Text
Archives of Blood Transfusion & Disorders
Existing Errors and Recommendations for Transfusion of Red Blood Assessment, Clinical Evaluation of Changes in Hematocrit
Andrey Belousov
Department of Anesthesiology, Intensive Care, Transfusiology and Hematology, Kharkiv Medical Academy of Postgraduate Education, Ukraine
*Corresponding author: Andrey Belousov, Laboratory Applied Nanotechnology of Belousov, Department of Anaesthesiology, Intensive Care, Transfusiology and Haematology, Kharkov Medical Academy of Postgraduate Education, Ukraine
Submission: April 04, 2018; Published: June 05, 2018
ISSN 2578-0239 Volume1 Issue3
Abstract
The focus of the article is rather situated on current faults and recommendations for transfusion of red blood assessment, clinical evaluation of changes in Hematocrit. The main task of therapy for acute massive blood loss is not urgent thoughtless transfusion of red blood cells for the fast recovery of the hemoglobin and Hematocrit levels. The oxygen-carrying capacity of blood does not directly reflect the delivery of oxygen to tissues. The severity of the patient’s condition depends of individual ability of the organism to resist hypoxia, mechanisms resulting in physiological compensation for the anemia caused by blood loss. The main tasks of therapy are timely maintaining appropriate and effective compensatory-adaptive reactions of an organism, providing of the sanogenetic processes. Quickly and comfortable algorithm assessment changes in Hematocrit was presented for used in practice. Objective analysis Hematocrit and hemoglobin levels should be carried out only in combination with data on blood pressure, pulse rate, respiratory rate, urine output and shock index.
keywordsHematocrit; Hemoglobin; Assessment system; Blood loss; Transfusion of red blood
Introduction
In modern health care today, blood transfusion plays a vital role. Blood transfusion can alleviate health and save life if used appropriately. According to WHO [1], appropriate use of blood products is defined as “the transfusion of safe blood products only to treat a condition leading to significant morbidity or mortality that cannot be prevented or managed effectively by other means”. RBC transfusions are used to treat hemorrhagic age and to improve oxygen delivery to tissues. Transfusion of RBCs should be based on the patient’s clinical condition. Indications for RBC transfusion include acute sickle cell crisis (for stroke prevention), or acute blood loss of greater than 1,500mL or 30 percent of blood volume. Patients with symptomatic anemia should be transfused if they cannot function without treating the anemia [2]. Symptoms of anemia may include fatigue, weakness, dizziness, reduced exercise tolerance, shortness of breath, changes in mental status, muscle cramps, and angina or severe congestive heart failure. The 10/30 rule - transfusion when a patient has a hemoglobin level less than or equal to 10g per dL (100g per L) and a Hematocrit level less than or equal to 30 percent - was used until the 1980s as the trigger to transfuse, regardless of the patient’s clinical presentation [2,3].
In 1999, a randomized, multicenter, controlled clinical trial evaluated a restrictive transfusion trigger (hemoglobin level of 7 to 9g per dL [70 to 90g per L]) versus a liberal transfusion trigger (hemoglobin level of 10 to 12g per dL [100 to 120g per L]) in patients who were critically ill. Restrictive transfusion practices resulted in a 54 percent relative decrease in the number of units transfused and a reduction in the 30 day mortality rate. The authors recommended transfusion when hemoglobin is less than 7g per dL, and maintenance of a hemoglobin level between 7 to 9g per dL [4]. A recently updated Cochrane review supports the use of restrictive transfusion triggers in patients who do not have cardiac disease [5].
A similar study was carried out in critically ill children. The restrictive transfusion trigger was a hemoglobin level of 7g per dL, with a target level of 8.5 to 9.5g per dL (85 to 95g per L). The liberal transfusion trigger was a hemoglobin level of 9.5g per dL, with a target level of 11 to 12g per dL (110 to 120g per L). Patients in the restrictive group received 44 percent fewer blood transfusions, with no difference in rates of multiple organ dysfunction syndrome or death. The restrictive transfusion strategy is useful for children who are stable patients in intensive care. It should not be used in preterm neonates or in children with severe hypoxemia, active blood loss, hemodynamic instability, or cyanotic heart disease [6]. Key recommendations for transfusion of red blood cells is presented in Table 1.
Table 1: Key recommendations for transfusion of red blood cells.
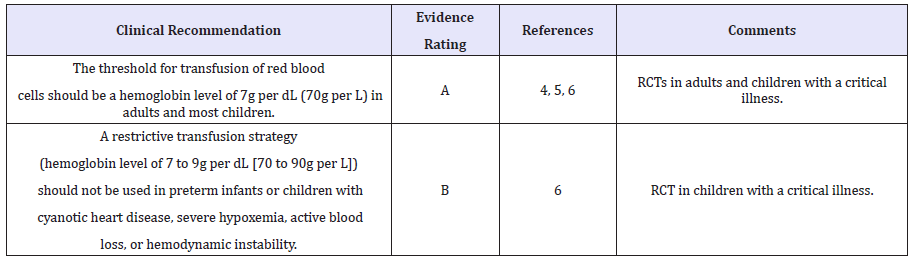
Notes: RCT = randomized controlled trial. A = consistent, good-quality patient-oriented evidence; B = inconsistent or limited-quality patient-oriented evidence. For information about the SORT evidence rating system.
Thus, many authors emphasis that hemoglobin and Hematocrit levels is important not only for transfusion of red blood cells, but is target indicates. The main purpose is on the contrary show that Hematocrit and hemoglobin levels not only does not reflects the severity of the patient with blood loss, but also a priori are not targeted indicators for transfusion of red blood cells. Clinical Transfusiology is new medical discipline which accumulates fundamental knowledge in Haematology, Immunohematology, Path physiology, Biochemistry, Biophysics, Histology fields and other adjacent medical specialties. Unfortunately, today exist erroneous and potentially dangerous point of view regarding the importance of this discipline among some anaesthesiologists and resuscitators. Inertia of the false traditional views on blood loss and an archaism of fundamental knowledge in the field of Clinical Transfusiology are influences on quality writing of the clinical protocols transfusion of red blood cells. Availability of large clinical trials, where absent information of mechanisms resulting in physiological compensation for the anemia caused by blood loss forms among physicians a false concept about adequate assessment severity of the patients’ clinical condition. Intensive care physicians and anaesthesiologists should be aware of treatment a priority action which maintains the compensatoryadaptive reactions of an organism in case severe blood loss. The primarily, the therapy must be directed at the elimination of acute deficit circulating blood volume, secondly, correction of coagulation factors, and only in the last instance - to a possible increase in the number of the oxygen-carrier [7]. The severity of the patient’s condition in acute massive blood loss does not depend directly on the hemoglobin and hematocritindicators.
At rest, there is a large reserve in oxygen delivery, since the rate of delivery normally exceeds consumption by a factor of four. Thus, if intravascular volume is maintained during bleeding and cardiovascular status is not impaired, oxygen delivery theoretically will be adequate until the Hematocrit falls below 10 percent because greater cardiac output, rightward shift of the oxygen-hemoglobin dissociation curve, and increased oxygen extraction can compensate for the decrease in arterial oxygen content. These predictions were confirmed in a study in which healthy resting individuals underwent acute isovolemic reduction of their hemoglobin to 5g/dL (equivalent to a Hematocrit of approximately 15 percent) [8]. Though some individuals did develop Electrocardiogram (ECG) changes consistent with myocardial ischemia, there was little evidence of inadequate oxygen delivery, and the fall in hemoglobin was associated with progressive increases in stroke volume and heart rate (and therefore cardiac output), and a progressive reduction in the systemic vascular resistance. Heart rate was found to increase linearly in response to the acute isovolemic anemia [9]. Of note, cognitive function measured by reaction time and immediate memory was impaired when the hemoglobin concentration was reduced to 5 to 6g/dL [10].
The oxygen-carrying capacity of blood does not directly reflect the delivery of oxygen to tissues [11]. The severity of the patient’s condition depends of individual ability of the organism to resist hypoxia, mechanisms resulting in physiological compensation for the anemia caused by blood loss [12]. As rule blood transfusion is usually administered to patients who are ill with underlying comorbidities, and there is concern that compensatory mechanisms may be impaired in critically ill patients, particularly in patients with underlying cardiovascular disease. Therefore, continuous improvement has become an important strategy in improving personalized express-diagnostic systems of functional state of the organism, estimation algorithms of the patient’s severity in case of blood loss [13]. Many hospitals have developed general guidelines for the appropriate use of blood transfusion, and an “implementation blueprint” for establishing a patient blood management program has been published by members of the High Value Practice Academic Alliance [14]. A patient blood management program uses “an evidence-based multidisciplinary approach to optimizing the care of patients who might need transfusion.” Patient blood management programs “include interventions taken early in the preparation of medical and surgical patients for treatment, as well as techniques and strategies in the preoperative, operative, and postoperative periods or completion of treatment” [15]. Three pillars of this type of program include optimizing haematopoiesis, minimizing blood loss and bleeding, and harnessing and optimizing tolerance of anemia [16]. I fully support this authors ‘ point of view. I’m in favour of such programs, because they attempt to reduce unnecessary transfusion and may reduce costs.
However, this programs and broad guidelines should not supersede clinical judgment in decisions regarding transfusion, especially by clinicians who are familiar with the individual patient. As an example, if a patient is experiencing symptoms that are known to reflect cardiac ischemia in that individual, transfusion may be appropriate. Alternatively, if a patient is known to tolerate a lower hemoglobin than that specified in the guideline, then it may be possible for that patient to avoid transfusion. Today one of the alternative variants of the objective clinical evaluation of patients can be PHUAS (Physiological Universal Analytic System) program [17].
The main task of therapy for acute massive blood loss is not urgent thought less transfusion of red blood cells for the fast recovery of the hemoglobin and Hematocrit levels. The main tasks of therapy are timely maintaining appropriate and effective compensatory-adaptive reactions of an organism, providing of the sanogenetic processes.
Table 2: The variants of changes in Hematocrit on the background of the basic organism physiological parameters
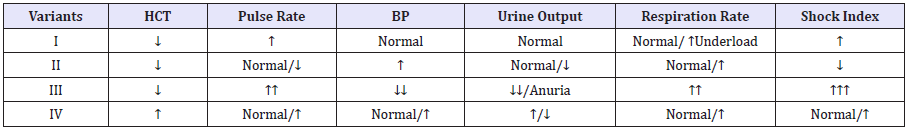
Notes: HCT – hematocrit; BP - blood pressure; Shock index = Pulse rate/ Systolic blood pressure (normal =0.54).
With regard to changes in Hematocrit and hemoglobin. Experienced practitioners know that a decrease in hematocrit and hemoglobin levels does not always reflect the degree of blood loss. For example, hem dilution is causes a decrease in Hematocrit and hemoglobin levels. On the contrary thickening of the blood is causes an increase in hemoglobin and hemoglobin levels. Examples of clinical of the variants of changes in Hematocrit on the background of the reaction of the basic organism physiological parameters were presented in Table 2. This table is quickly and comfortable algorithm assessment changes in Hematocrit which need used in practice.
Discussion
Variant I
Low Hematocrit levels against the background of moderate tachycardia and tachypnea, that increase after physical activity. Blood pressure and hourly urine output are normal. The shock index is moderately elevated. This variant reduction in hematocrit characterizes the hemic hypoxia. In this case, when absence of the dynamic reduction in hemoglobin and hematocrit levels transfusion of red blood cells is not advisable.
Variant II
Low Hematocrit levels against the background of normal or slight decrease in heart rate. Blood pressure up. Urine output - decreased or normal. The shock index is always below normal (< 0.54). This variant characterizes the hypovolemia state. At the same time transfusion of red blood cells in order to increase the level of Hematocrit is not only unjustified but also danger to the patient’s life due to volume overload of the small circle of blood circulation.
Variant III
Low Hematocrit levels against the background of expressed symptoms of tachycardia and tachypnea. Decreased blood pressure and hourly urine output to anuria. The shock index is elevated (>1.5). This variant characterizes the mixed form of hypoxia (circulatory + hemic hypoxia) that is caused by massive blood loss.
Variant IV
High Hematocrit levels against the background of normal or high heart rate, increased blood pressure and urine output. At the same time dynamics of increased in Hematocrit may be accompanied by decreased hourly urine output. The shock index is elevated. This variant characterizes the hypovolemia polycythemia.
Conclusion
Thus, Hematocrit and hemoglobin levels not only do not objectively reflect degree of blood loss and patient’s severity but also a priori are not targeted indicators for transfusion of red blood cells. Objective analysis Hematocrit and hemoglobin levels should be carried out only in combination with data on blood pressure, pulse rate, respiratory rate, urine output and shock index. Recommendations for transfusion of red blood cells which are based only on Hematocrit and hemoglobin data are not justified and unsafe for the patient.
References
- World Health Organization (2001) The clinical use of blood in obstetrics, pediatrics. Surgery & Anaesthesia, Trauma & Burns, Blood Transfusion Safety.
- Klein HG, Spahn DR, Carson JL (2007) Red blood cell transfusion in clinical practice. Lancet 370(9585): 415-426.
- Ferraris VA, Ferraris SP, Saha SP, Bennett-Guerrero E, Hill SE, et al. (2007) Perioperative blood transfusion and blood conservation in cardiac surgery: The Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg 83(5 Suppl): S27-S86.
- Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, et al. (1999) A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 340(6): 409-417.
- Carless PA, Henry DA, Carson JL, Hebert PP, McClelland B, et al. (2010) Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev (10): CD002042.
- Lacroix J, Hébert PC, Hutchison JS (2007) TRIPICU Investigators; Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators Network. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 356(16): 1609-1619.
- Belousov A, Malygon E, Yavorskiy V (2017) Calculating the True Volume of Blood Loss. Journal of Anesthesia & Clinical 8(11): 2017.
- Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, et al. (1998) Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 279(3): 217-221.
- Weiskopf RB, Feiner J, Hopf H, Viele MK, Watson JJ, et al. (2003) Heart rate increases linearly in response to acute isovolemic anemia. Transfusion 43(2): 235-240.
- Weiskopf RB, Kramer JH, Viele M, Neumann M, Feiner JR, et al. (2000) Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology 92(6): 1646-1652
- Nielsen ND, Martin-Loeches I, Wentowski C (2017) The effects of red blood cell transfusion on tissue oxygenation and the microcirculation in the intensive care unit: a systematic review. Transfus Med Rev 31(4): 205-222.
- Pope A, French G, Longnecker DE (2017) Fluid Resuscitation: State of the Science for Treating Combat Casualties and Civilian Injuries. Institute of Medicine (US) Committee on Fluid Resuscitation for Combat Casualties. National Academies Press, USA.
- Jeffrey L Carson, Steven Kleinman MD (2018) Indications and hemoglobin thresholds for red blood cell transfusion in the adult.
- Sadana D, Pratzer A, Scher LJ, Saag HS, Adler N, et al. (2018) Promoting high-value practice by reducing unnecessary transfusions with a patient blood management program. JAMA Intern Med 178(1): 116-122.
- www.aabb.org/resources/bct/pbm/Documents/best-practices-pbm. pdf
- Shander A, Van Aken H, Colomina MJ, Gombotz H, Hofmann A, et al. (2012) Patient blood management in Europe. Br J Anaesth 109(1): 55- 68.
- Belousov A (2015) New Program for Estimation of Degree of the Patient’s Severity. Journal of Anesthesia and Surgery. Ommega Publisher. NJ-USA 2(2): 1-3.
© 2018 Andrey Belousov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






