- Submissions

Full Text
Archives of Blood Transfusion & Disorders
Platelets from Patients with Hereditary Dense- Granule Disorders Support Surface-Associated Factor XII Activation
Natalia V Zakharova1,2*, Nadezhda A Podoplelova1, Herve Chambost3, Alexei L Drobyshev4 and Irina A Demina1
1Federal Research and Clinical Centre of Paediatric Haematology, Oncology and Immunology (Rogachev Centre), Russia
2Emanuel Institute for Biochemical Physics, Russian Academy of Sciences, Russia
3Aix Marseille Université, 13005 Marseille, France
4Centre for Theoretical Problems of Physicochemical Pharmacology, Russia
*Corresponding author: NV Zakharova, Federal Research and Clinical Centre of Paediatric Haematology, Oncology and Immunology, 1 Samory Mashela Str, 117997 Moscow, Russia
Submission: August 22, 2017 Published: October 16, 2017
ISSN: 2578-0239Volume1 Issue1
Abstract
Identifying the mechanisms underlying the contribution of coagulation factor XII (fXII) to arterial thrombosis is of particular clinical interest. The physiological importance of platelet dense granules-derived polyphosphates in fXII activation is a subject of intense discussion and controversy. Our previous study suggested that the potently activated platelet-surface plays the more essential role in fXII activation. We, therefore, assayed the plasma fXII activation by potently stimulated platelets from a variety of patients with inherited granule disorders to clarify the possible role of surfaceassociated dense- or α-granule components. The assay was performed with A23187-stimulated platelets washed from secretions containing fXII(a) inhibitors. Platelets from two patients with dense granule defects and one patient with Wiskott-Aldrich syndrome supported surface-associated fXII activation significantly better than platelets from 52 healthy donors. The platelets from two patients with Hermansky-Pudlak syndrome demonstrated no significant difference compared with the donor group. In contrast, platelets from one patient with α,δ-storage pool deficiency as well as platelets from four patients with gray platelet syndrome of different genetic backgrounds did not support fXII activation. The results underscore the non-essential role of dense granule components in platelet surface-associated fXII activation.
Introduction
Coagulation factor XII (fXII) is a key component of the blood contact system and is involved in a number of vascular pathophysiologic processes in addition to coagulation [1]. Several recent findings suggest that fXII may play an important role in arterial thrombosis without affecting haemostasis. Of note, thrombus formation is impaired in fXII-deficient mice [2-4] and is suppressed in wild type mice and primates following fXIIa inhibition [5,6].
The mechanism of fXII contribution to arterial thrombosis is presently unclear. Platelets have been considered as probable activators of fXII in arterial thrombi. Several studies have suggested different methods of platelet-related fXII activation, namely via the platelet surface [7-10], by platelet-derived and circulating plasma microparticles [11,12] and via platelet dense granules-derived polyphosphates (poly-P) [13] (Supplementary Table S1). Recently, poly-P have been widely considered to be the most important platelet-derived fXII activators [14-18]. However, a number of studies have shed doubt on the overall role of platelets and the importance of platelet-secreted poly-P fXII activation [19-24]. Our results suggest that fXII activation in plasma by platelet-derived substances may be counteracted by the simultaneous secretion of platelet-derived fXIIa inhibitors [10] such as C1 inhibitor [25] or histidine-rich glycoprotein [26,27]. Conversely, the potently stimulated platelets washed from secretions resulted in significant surface-associated fXII activation sufficient for triggering the clotting of plasma, a process that was most likely related to surfaceassociated α-granule components [10].
Accordingly, we assayed the surface-associated fXII activation by potently stimulated platelets from a variety of patients with inherited granule disorders and 52 healthy donors. Patients included two patients with dense granule defects (DGD), two patients with Hermansky-Pudlak syndrome (HPS), one patient with Wiskott-Aldrich syndrome (WAS), one patient with α,δ-storage pool deficiency (α,δ-SPD), and four patients with gray platelet syndrome (GPS, α-granule deficiency) from different genetic backgrounds. The activated platelets were washed from their secretions, and we only assayed the platelet surface-associated reaction.
Materials and Methods
Materials
The following materials were from the sources shown in parentheses: fXII (Enzyme Research Laboratories; South Bend, IN, USA); FITC-annexin V (BD Biosciences; San Jose, CA, USA); prostaglandin E1 (PGE1, MP Biochemicals; Irvine, CA, USA); chromogenic substrate S2302 (Chromogenix; Milano, Italy); HEPES, bovine serum albumin, apyrase grade VII, mepacrine (quinacrine), EDTA and DMSO (Sigma-Aldrich; St Louis, MO, USA); calcium ionophore A23187 (Tocris Bioscience; Ellisville, MO, USA). Factor XIIa-specific corn trypsin inhibitor (CTI) was kindly provided by Dr. Smolyaninov (Institute of Protein Research, Russian Academy of Sciences, Pushchino, Russia).
Blood collection, platelet isolation and plasma preparation
All procedures involving blood collection were performed in accordance with the Declaration of Helsinki using a protocol approved by the institution’s Ethics Committees of Federal Research and Clinical Centre of Paediatric Haematology, Oncology and Immunology, Aix Marseille University, and the Centre for Theoretical Problems of Physicochemical Pharmacology. Written informed consent was obtained from all donors and patients. Blood was collected into 106mM sodium citrate at 9:1 volume ratio and supplemented with apyrase (0.1U/ml) and PGE1 (1μM). After precipitation from platelet-rich plasma at 400g for 5min, platelets were resuspended and washed with buffer A (150mM NaCl, 2.7mM KCl, 1mM MgCl2, 0.4mM NaH2PO4, 20mM HEPES, 5mM glucose, and 0.5% bovine serum albumin, pH 7.4). To prepare chelated plasma for the fXII activation assay, after platelet precipitation, the plasma was further centrifuged for 30min at 16,000g to remove microparticles [28], diluted to 40% with buffer A, and supplemented with 8mM sodium EDTA (pH 7.6). Calcium chelation was necessary to exclude thrombin generation and fXIIa-independent reactions in a further chromogenic assay.
Patients
Dense granule defects (DGD): Two patients with DGD were assayed at the La Timone Hospital in Marseille: a 70-year-old man (DGD-1) and a 50-year-old woman (DGD-2). These two patients had been previously examined on several occasions for bleeding episodes. They had normal platelet counts and aggregation and exhibited a profound decrease in two dense granule substances, serotonin (0.16 and 0.17 AU-Normal range: 0.30-1.20) and granulophysin (CD63) (mean fluorescence intensity: 1.30 and 1.22- Normal range: 1.50-4.23). Whole mount and thin section platelet transmission electron microscopy revealed a high percentage of platelets without dense granules (52 and 80% - normal value <30%). DGD-2 was found to carry an FLI-1 mutation. The both DGD-1 and DGD-2 platelets showed low capture and release of α-granule marker mepacrine.
Hermansky-Pudlak syndrome (HPS): Two patients with HPS of different origin were assayed: a 49-year-old woman at the La Timone Hospital in Marseille (HPS-1) and a 30-year-old woman at the Rogachev Centre in Moscow (HPS-2). Both patients had characteristic laboratory and clinical features of HPS that included a long bleeding time, albinism, nystagmus, pulmonary or mammary glandular fibrosis, and anaemia. HPS-1 platelets lacked dense granules as determined by electron microscopy, had a low CD63 expression upon stimulation, and had decreased aggregation in response to ADP and collagen. The both HPS-1 and HPS-2 platelets showed low capture and release of δ-granule marker mepacrine.
Wiskott-Aldrich syndrome (WAS): One patient with WAS, a 12-year-old boy at the Rogachev Centre in Moscow, possessed an X chromosome gene WAS mutation in exon 1 (AGT-TGT c 4A>T, p Ser2Cys; and Hemi, intron insertion c 273+(5+11)) and had a familial history with similar clinical patterns that was inherited only by the men in the family. These clinical manifestations included epistaxis, petechiae, multiple ecchymoses, and subretinal macular haemorrhage. His platelets showed critical functional depression, decreased activated integr in αIIbβ3 exposition (PAC-1 mean fluorescence intensity 560, normal range 700-1500), low release of α-granules (CD62P mean fluorescence intensity 3730, normal range 10660-18640) and decreased capture of δ-granule marker mepacrine by non-activated platelets (mean fluorescence intensity 480, normal range 770-1360).
α,δ-Storage pool deficiency (α,δ-SPD): One patient with α,δ- SPD, a 6-year-old girl at the Rogachev Centre in Moscow, had inborn thrombocytopenia, signs of megakaryocyte-erythroid dyspoiesis, slight hypocoagulation on the APTT test, and lack of ristocetincofactor activity with moderately-decreased vWF-antigen content. Her platelets showed slightly decreased aggregation in response to ADP and ristocetin and were significantly deficient in their ability to release both α-granules (CD62P mean fluorescence intensity 5370, normal range 10660-18640) and δ-granules (mepacrine mean fluorescence intensity 1410 and 1140 for non-activated and activated platelets, respectively, at normal ranges 770-1360 and 210-260, respectively).
Gray platelet syndrome (GPS): One of the four assayed patients with recessive gray syndrome was a 28-year-old woman at the La Timone Hospital in Marseille (GPS-1). Morphological platelet examination and intragranular protein measurements confirmed the paucity of platelet α-granules. The serum PAI-1 antigen level was 3ng/ml (normal value: 92-283) indicating a complete absence of this alpha granule protein. Sanger sequencing revealed a homozygous mutation in the NBEAL2 gene (c7202 ins TCCTTCATCAC), which is known to play a role in the formation of the α-granule [29].
Three other patients with GPS at the Rogachev Centre in Moscow were relatives with an autosomal dominant familial version of the syndrome: a 36-year-old man [10] (GPS-2), his 4-year-old son (GPS-3), and his 2-year-old daughter (GPS-4). They had characteristic laboratory and clinical features of GPS: severe α-granule protein deficiencies, large, agranular and grey platelets, moderate thrombocytopenia, recurrent ecchymosis, epistaxis, and decreased aggregation in response to ADP, but not to ristocetin. The diagnosis was based on the presence of grey platelets on stained smear samples, and a critical decrease in alpha-granular markers (P-select in, factor V and fibrinogen).
Platelet activation and secretion removal
Platelets were activated at a concentration of 2×108/mL for 10min with 10μM A23187 in the presence of 2.5mM CaCl2. This activation typically yields approximately 90% of PS-positive poorly aggregated platelets [10]. To remove the soluble secreted components including fXII(a) inhibitors [25-27], the activated platelets were further washed with buffer A through double precipitation at 16,000g for 10min.
Platelet-dependent fXIIa generation
Platelet-dependent fXIIa generation was measured with the chromogenic substrate S2302 as described [10]. Briefly, the platelets were stimulated with A23187, washed from the secretions, and mixed at 1×107/mL with 200μM S2302 and 20% chelated plasma (as fXII source). For the controls, the platelets were replaced with buffer A. Previously, the activity of other S2302 cleaving factors had been shown to be negligible under these conditions [10]. The results were expressed as concentrations of the fXIIa formed, which were calculated using the determined 1nM fXIIa reaction rate value. The plasma-related fXII auto-activation in most cases under these conditions ranged from 0.4 to 0.7nM (Supplementary Figure S1A), while the standard error for the individual measurements was ±0.07nM (for at least three replicates). Therefore, the reference plasma-related fXII activation was estimated in each particular experiment and subtracted from the individual values obtained for this plasma with added platelets (Supplementary Figure S1B). The results presented below correspond to platelet dependent fXIIa formation only, while the platelet independent plasma-related fXII auto-activation was considered as ‘zero’ level (0±0.07 nM). The cases with negative values that were significantly different from zero implicated the platelet-dependent inhibition of plasma autoactivated fXIIa.
Statistics
The values for each individual were calculated as the mean values from at least three independent measurements. Statistical significance was estimated with the non-parametric two-tailed Mann-Whitney test for the groups of patients or was referenced to the empirical distribution among individual healthy donors as a two-tailed value.
Results and Discussion
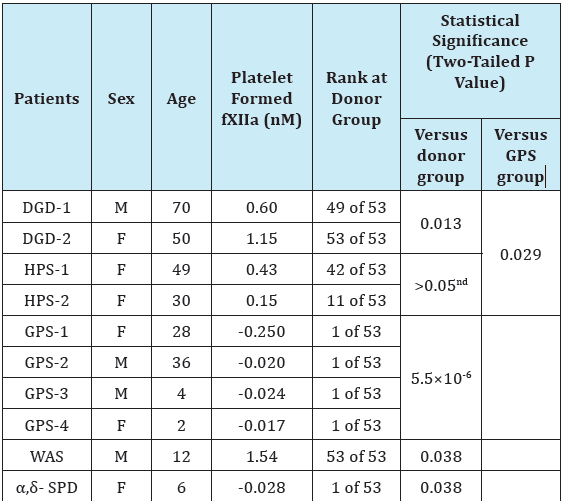
Table 1 illustrate the platelet surface-associated fXII activation for different patients in comparison with healthy donors. The results are shown in accordance to different dense- and/or α-granule disorders. The data indicate that platelets from two patients with dense granule defects (DGD) paradoxically supported fXII activation significantly better than platelets from healthy donors (n=2, p=0.013). Interestingly, the activation was essentially higher in the case of platelets from DGD-2 patient that had the higher percentage of platelets without dense granules (80% versus 52% in the case of DGD-1). However, the platelets from patients with Hermansky-Pudlak syndrome (HPS) demonstrated no significant difference from the donor group (Table 1). Nevertheless, fXII activation was reliably present for all of the platelets that lacked the dense granules only. This strongly suggested that dense granule secretion is not essential for surface-associated fXII activation.
The fXII activation by platelets from patients with HPS seems to contradict the earlier results that suggest that PAR-1 stimulation of HPS-platelets fails to trigger fXII activation in PRP because of impaired poly-P secretion [13]. However, unlike earlier studies, the current study considered platelet surface-associated fXII-activation [13], applied potent platelet stimulation, and accounted for the possible fXIIa inhibition by platelet-secreted material [10,25,26]. Thus, our results are rather specific in their evaluation of the capacity of the platelet surface to trigger fXII activation.
The earlier finding regarding the poor efficiency of gray platelets to activate fXII suggests that the membrane-associated α-granule components must be playing an important role [10]. The results obtained here with platelets from additional patients with GPS were consistent with earlier ones: in all cases, the values were lower than the minimum from the donor group Table 1. Importantly, the direct comparison of results divided into the groups consisting of dense- or α-granule disorders supported that the group with dense-granule disorders (including the results for DGD and HPS) was significantly different from the GPS group with α-granule deficiency (p=0.029, Table 1). This suggests the superior importance of α-granule components in membrane-associated fXII activation relative to the components of dense-granules. The intriguing results were obtained with platelets from patients with Wiskott-Aldrich syndrome (WAS) and α,δ-storage pool deficiency (α,δ- SPD) Table 1. While the both platelet preparations had essentially decreased release of the both α- and dense granules, they demonstrated the opposite capacities in surface-associated fXII activation. The SPD-platelets did not activate fXII similar to GPSplatelets, however WAS-platelets unexpectedly demonstrated the highest fXII activation among all the analysed platelet preparations.
Because this study focused on the platelet surface-associated processes only, we used A23187-stimulation since it typically yields >90% PS-positive poorly aggregated platelets. In addition, the platelets could be easily washed away from the secreted material [10]. Importantly, this type of stimulation under these conditions is similar to platelet activation by potent physiological activators with respect to the triggering of surface-associated fXII activation [10].
Notably, the capacity for fXII activation was extremely variable within the donor group. In particular, the two lowest donor values in Supplementary Figure S1B were <0.05nM fXIIa formed (upon the measurement accuracy ±0.07nM), while the two highest values exceeded 0.75nM (that is ~1.5 times higher than the average plasma-related fXII auto-activation). The cause of this variation is unclear and may be of clinical interest and an interesting topic for further research. However, we did not account for the possible retention of α-granule-derived fXIIa inhibitors by platelet surfaces under these conditions [25,30], although the secreted inhibitors were removed. It is likely that the quantity of surface-associated inhibitors varies among individuals. The values obtained for GPS and SPD platelets that were lying below the ‘zero’ level (Figure S1, Table 1) also suggest a slight inhibition of plasma auto-activated fXIIa by these platelets. However α-granule-secretion was impaired in these cases, which suggests that platelets may also retain non-α- granule inhibitors.
Figure S1: The cumulative distributions for plasmaand platelet-related fXII activation. (A) The cumulative distributions for the plasma-related fXII auto-activation (for 20% chelated plasma preparations, n=60). The dashed frame outlines the area within one standard deviation from the average (16-85% suggest the Gaussian distribution). (B) The cumulative distributions for the platelet-related fXII activation after deduction of plasma-related auto-activation (for A23187-stimulated platelets from 52 healthy donors). As the distribution was apparently asymmetrical (non-Gaussian), the statistical significance was further estimated with the nonparametric two-tailed Mann-Whitney test.
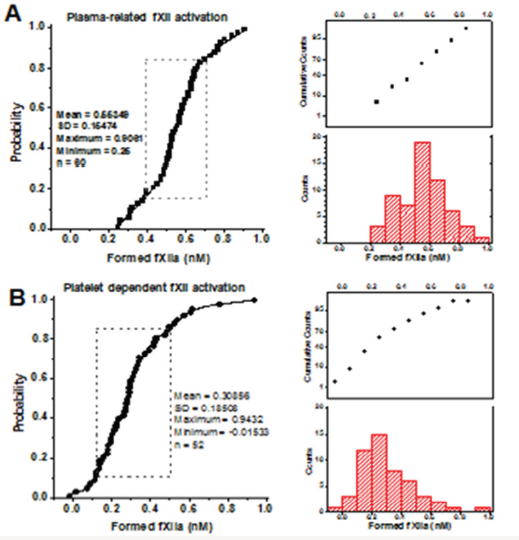
Table 1: Factor XII activation by platelets from patients with dense- and/or α- granule disorders.
ndNot significantly different.
Table S1: Studies considering platelet-related fXII activation.
Overall, it can be concluded that the platelets from patients with dense granule disorders still support fXII activation. The results additionally suggest that α-granule release is more essential in platelet surface-associated fXII activation than release of dense granules. The increased fXII activation by DGD and WAS platelets as well as divergence of results for SPD and WAS platelets seem to be intriguing and point to insufficient understanding of the regulatory function of platelets in the contact processes. Further analysis of platelets from additional patients will help to clarify the relationship between dense granule disorders and increased fXII activation. The particular role of membrane-associated α-granule components in fXII activation also requires further investigations.
Acknowledgment
We thank MA Panteleev, Dr. MC Alessi, EN Lipets, and YN Kotova for constructive discussion and critical review of the manuscript; consulting physicians MA Kumskova, PA Zharkov, IN Smirnova, and EA Likhacheva for generously providing patient blood samples. This study was supported by the Russian Foundation for Basic Research grant 16-04-00125.
References
- Colman RW, Schmaier AH (1997) Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood 90(10): 3819-3843.
- Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, et al. (2010) A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood 116(19): 3981-3989.
- Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, et al. (2005) Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med 202(2): 271-281.
- Kuijpers MJE, Van Der Meijden PEJ, Feijge MAH, Mattheij NJA, May F, et al. (2014) Factor XII Regulates the Pathological Process of thrombus Formation on Ruptured Plaques. Arterioscler Thromb Vasc Biol 34(8): 1674-1680.
- Hagedorn I, Schmidbauer S, Pleines I, Kleinschnitz C, Kronthaler U, et al. (2010) Factor XIIa inhibitor recombinant human albumin Infestin-4 abolishes occlusive arterial thrombus formation without affecting bleeding. Circulation 121(13): 1510-1517.
- Matafonov A, Leung PY, Gailani AE, Grach SL, Puy C, et al. (2014) Factor XII inhibition reduces thrombus formation in a primate thrombosis model. Blood 123(11): 1739-1746.
- Castaldi PA, Larrieu MJ, Caen J (1965) Availability of platelet Factor 3 and activation of factor XII in thrombasthenia. Nature 207(995): 422-424.
- Walsh PN, Griffin JH (1981) Platelet-coagulant protein interactions in contact activation. Ann N Y Acad Sci 370: 241-252.
- Back J, Sanchez J, Elgue G, Ekdahl KN, Nilsson B (2010) Activated human platelets induce factor XIIa-mediated contact activation. Biochem Biophys Res Commun 391(1): 11-17.
- Zakharova NV, Artemenko EO, Podoplelova NA, Sveshnikova AN, Demina IA, et al. (2015) Platelet Surface-Associated Activation and Secretion Mediated Inhibition of Coagulation Factor XII. PLoS ONE 10(2): e0116665.
- Van Der Meijden PE, Van Schilfgaarde M, Van Oerle R, Renne T, ten Cate H, Spronk HM (2012) Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost 10(7): 1355-1362.
- Lipets E, Vlasova O, Urnova E, Margolin O, Soloveva A, et al. (2014) Circulating contact-pathway-activating microparticles together with factors IXa and XIa induce spontaneous clotting in plasma of hematology and cardiologic patients. PLoS One 9(1): e87692.
- Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, et al. (2009) Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 139(6): 1143-1156.
- Renne T, Schmaier AH, Nickel KF, Blomback M, Maas C (2012) In vivo roles of factor XII. Blood 120(22): 4296-4303.
- Mackman N, Gruber A (2010) Platelet polyphosphate: an endogenous activator of coagulation factor XII. J Thromb Haemost 8(5): 865-867.
- Puy C, Tucker EI, Wong ZC, Gailani D, Smith SA, et al. (2013) Factor XII promotes blood coagulation independent of factor XI in the presence of long-chain polyphosphates. J Thromb Haemost 11(7): 1341-1352.
- Verhoef JJF, Barendrecht AD, Nickel KF, Dijkxhoorn K, Kenne E, et al. (2017) Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood 129(12): 1707-1717.
- Long AT, Kenne E, Jung R, Fuchs TA, Renne T (2016) Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost 14(3): 427-437.
- Faxalv L, Boknas N, Strom JO, Tengvall P, Theodorsson E, et al. (2013) Putting polyphosphates to the test: evidence against platelet-induced activation of factor XII. Blood 122(23): 3818-3824.
- Boknas N, Faxalv L, Strom JO, Tengvall P, Theodorsson E, et al. (2014) Response: Platelets do not generate activated factor XII-how inappropriate experimental models have led to misleading conclusions. Blood 124(10): 1692-1694.
- Smith SA, Choi SH, Davis Harrison R, Huyck J, Boettcher J, et al. (2010) Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood 116(20): 4353-4359.
- Panteleev MA, Dashkevich NM, Ataullakhanov FI (2015) Hemostasis and thrombosis beyond biochemistry: roles of geometry, flow and diffusion. Thromb Res 136(4): 699-711.
- Chelushkin MA, Pamteleev MA, Sveshnikova AN (2017) Activation of the contact pathway of blood coagulation on the circulating microparticles may explain blood plasma coagulation induced by dilution. Biochemistry (Moscow), Supplement Series A: Memebrane and Cell Biology 11(2): 130-143.
- Danese E, Montagnana M, Lippi G (2016) Factor XII in Hemostasis and Thrombosis: Active Player or (Innocent) Bystander? Semin Thromb Hemost 42(6): 682-688.
- Schmaier AH, Amenta S, Xiong T, Heda GD, Gewirtz AM (1993) Expression of platelet C1 inhibitor. Blood 82(2): 465-474.
- Leung LL, Harpel RL, Nachman RL, Rabellino EM (1983) Histidine-rich glycoprotein is present in human platelets and is released following thrombin stimulation. Blood 62(5): 1016-1021.
- MacQuarrie JL, Stafford AR, Yau JW, Leslie BA, Vu TT, et al. (2011) Histidine-rich glycoprotein binds factor XIIa with high affinity and inhibits contact-initiated coagulation. Blood 117(15): 4134-4141.
- Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, et al. (2007) Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost 97(3): 425-434.
- Albers CA, Cvejic A, Favier R, Bouwmans EE, Alessi MC, et al. (2011) Exome sequencing identifies NBEAL2 as the causative gene for Gray Platelet Syndrome. Nat Genet 43(8): 735-737.
- Schmaier AH, Smith PM, Colman RW (1985) Platelet C1- inhibitor. A secreted alpha-granule protein. J Clin Invest 75(1): 242-250.
© 2017 Natalia V Zakharova, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






