- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Antibiotic Sensitivity Pattern of Bacteria Isolated from Semen of Male Patients with Infertility Attending Murtala Muhammad Specialist Hospital Kano, Nigeria
Nasiru A Salisu1, Farouk S Nas2, Sani U Diso3 and Muhammad Ali4*
1 Department of Medical Laboratory Science, Bayero University,Nigeria
2 Department of Biological, Bayero University, Nigeria
3 Department of Pharmaceutical Technology, Nigeria
4 Department of Microbiology, Kano University of Science and Technology Wudil, Nigeria
*Corresponding author: Muhammad Ali, Microbiology Department, Kano University of Science and Technology Wudil Kano, Nigeria
Submission: June 11, 2018; Published: July 18, 2018

ISSN 2640-9275Volume1 Issue4
Abstract
The study was aimed tocharacterized and determinethe antibiotic sensitivity pattern of bacteria isolated from semen of male patients with infertility attending Murtala Muhammad Specialist Hospital (MMSH) Kano, Nigeria. Two hundred (200) Semen specimens were collected from males with infertility attending the clinic and General out Patient Department of MMSH Kano. The seminal fluids were diluted with sterile saline, centrifuged and cultured on Nutrient agar, Blood agar, Chocolate agar and MacConkey agar then incubated aerobically and in 5% CO2at 37°C for 24 hours for the isolation of pathogenic microorganism. Isolates were identified based on Gram’s staining, biochemical tests and API 20E Test. The result shows a total of seventy six (76) isolates were obtained, Staphylococcusaureus was found to have the highest occurrence of 31 (40.79%), whereas the least was found to be Mycoplasma species, 1(1.32%). Other microorganisms encountered include; Coagulase negative Staphylococcus species14 (18.42%), Escherichia coli 11(14.47%),Klebsiella pneumonia 6(7.89%), Proteus mirabilis 5(6.58%),Pseudomonas aeruginosa 6 (7.90%) andNeisseria gonorrheae2(2.63%). The isolates were most sensitive to ciprofloxacin and ofloxacin. Ceftrizone and cefuroxime however showed superior activity against most of the isolates. The activity of the Tetracycline and Augumentin against almost all the isolates was not appreciable. Cotromethazole and Gentamicin showed moderate activity against most of the isolates and no activity was observed on Mycoplasma and Neisseria gonorrhoeae against most of the antibiotics.
Keywords: Antibiotics; Bacteria;Infertility;Semen;Sensitivity pattern
Introduction
The emergence of bacterial antimicrobial resistance has made the choice of empirical therapy more difficult and expensive [1]. Hence there is need for regular screening of organisms causing various infections and to characterize their antimicrobial susceptibility pattern to commonly used antibiotics at the hospital, regional, national and global levels to guide the clinicians to select a relevant antimicrobial for empirical treatment of infections. Infertility is the inability of a sexually active, non-contraception couple to achieve spontaneous pregnancy in one year. About 15% of couples do not achieve pregnancy within one year and seek medical treatment for infertility [2]. One in eight couples encounters problems in attempting to conceive a first child and one in six when attempting to conceive a subsequent child. Both sexes are more or less equally involved in infertility problem [3]. Men either alone or along with theirfemale partners, contribute to 40–45% cases of infertility[ 4]. Furthermore, infectious aetiology involving bacteria, virus, fungi, and protozoa contribute to 15% of male factor infertility [5].
Microbial infections of the genital tracts or semen are major causes of male infertility [6,7]. According to World Health Organization (WHO) [2], semen consists of concentrated suspension of spermatozoa and the fluid secreted by the accessory sex organs namely prostate gland, seminal vesicles, bulbourethral glands, and epididymides. The fluid secretion is about 90% of semen volume and dilutes the concentrated epididymal spermatozoa at ejaculation. Since the ejaculate is a mixture of secretions derived from the urogenital tract and the male accessory glands, seminal culture identifies the presence of germs in any section of the seminal tract [8].
Male urogenital tract infections are one of the most important causes of male infertility worldwide [9]. Askienazy-Elnhar[10] reported that genital tract infection and inflammation have been associated to 8-35% of male infertility cases and male accessory sex glands infection is a major risk factor in infertility [5]. Studies have also shown that when the characteristics of semen infected and uninfected were compared, semen with micro-organisms had poor indices of fertility Sanocka-Meclejeskaet al.[11]. Infertility affecting couples around the World is both a medical as well as social problem particularly in Nigeria [12]. Evidences are being accumulating on the association of asymptomatic bacteriospermia and altered semen quality [13]. There is difference as to the influence of certain microbial infection on male infertility [14]. Urinary tract infections are common in men and clinicians working with infertility frequently encounter patients with these diseases [15]. More than 90% of male infertility cases is due to either low sperm count (oligospermia), no sperm at all (azoospermia) or poor seminal fluid quality or combination of the two and this claimed to the increase prevalence of sexually transmitted diseases (STDs) and urogenital infections alarmed since 1992 [16]. Previous studies have identified Staphylococcus aureus, E.coli, Citrobacterspecies, Klebsiellaspecies, Pseudomonas aeruginosa, Proteus vulgaris, and Proteus mirabilis with infertility in male partners of infertile couples [7,9,17]. The study was aimed todeterminethe antibiotic sensitivity pattern of bacteria isolated from semen of male patients with infertility attending Murtala Muhammad specialist hospital Kano, Nigeria
Materials and Methods
ethical clearance
An approval (MOH/off/797/T.I/49) for the study was obtained from Research and Ethic committee Kano state ministry of health. The aim of the study was explained clearly to the clients and informed consent obtained before proceeding to the study.
study area
The study was conducted at Microbiology Department of Murtala Muhammad Specialist Hospital (MMSH), Kano. Kano state is located in the North-west Nigeria with coordinates 11030 N 8030 E. It shares borders with Kaduna state to the south- west, Bauchi state to the South-East, Jigawa state to the East, Katsina state to the West and Niger republic to the North. It has a total area of 20,131km2 (7,777sqm) and population of 11,058,300 [18].
sample collection
Semen specimens were collected from males with infertility attending the clinic and GOPD of MMSH Kano. The samples were collected from patients who have had 3-7 days of sexual abstinence from intercourse preferably by masturbation into a sterile clean wide-mouth container. Upon collection, samples were transferred without any delay to the Microbiology Department of MMSH in a nearly as possible to body temperature by placing the container inside a flask containing water at 33-37°C. Time of collection to the time the samples were received in the laboratory was recorded which must not exceed 45 minutes.Furthermore, analyses of samples with SQA machine were conducted at Microbiology Department, Aminu Kano teaching hospital (AKTH), Kano.
Culture
Culture of seminal fluid samples was done in aseptic condition within one hour of collection in accordance with WHO [2]. The seminal fluids were diluted with sterile saline (1:10) and centrifuged at1500 r.p.m. for 15min. After removing the supernatant, the sediments were cultured using 10μl calibrated loops on Nutrient agar, Blood agar, Chocolate agar and MacConkey agar which were incubated aerobically and in 5% CO2 at 37°C for 24 hours for the isolation of pathogenic microorganism [2]. Seminoculture were considered positive when the number of colonies was ≥104CFU/ ml for Gram positive cocci and ≥105CFU/ml for Gram negative rods [19].
Isolation and identification of isolates
Nutrient agar plates were prepared for the isolation of pure colonies from the primary plates. A colony was picked and streaked on nutrient agar plates and then incubated at 37°C for 24 hours. Using a sterile wire loop, a colony from each plate was picked and prepare for Gram’s staining and other biochemical tests, some of which include Catalase, Coagulase, DNAse, Triple iron agar, Oxidase and API 20E Test [20].
Gram’s Stain
The smear was made from the isolate on a clean grease free slide and allowed to air dried and fix. The smear was flooded with crystal violet as a primary stain and was allowed to stain for 2 minutes and rinsed with water. A mordant (lugol’s iodine) was then flooded and allowed to stay for 1 minute and rinsed with water. A smear was then flooded with secondary stain (neutral red) and was allowed to stain for 2 minutes and then rinsed in water and allowed to air dried [21].
Catalase test
Few colonies of the test organisms were emulsified in distilled water on a clean slide. 1-2 drops of 3% H2O2 were added and cover with cover slip [20].
Coagulase test
A drop of plasma was placedon a clean dried slide. A drop of saline was place next to the drop of plasma as control. With a loop a portion of the isolated colonies were emulsified in each drop starting with the saline until a smooth suspension was obtained. The suspension was then mixed well, and rocked gently for 5-10 seconds [20].
Dnase test
Using a sterile loop, test and control organism (ATCC 2923) were spot-inoculated and incubated at 35-37 overnight. The surface of plate was covered with 1mol/ml hydrochloric acid solution and excess was tipped off. Clearing around each colony was observed within 5 minutes of adding the acid [20].
Oxidase test
A piece of filter paper was moisture with substrate (1% tetramethyle–p-phenylene-diamine dihydrochloride). A wooden stick was used to remove small portion of bacterial colony and streak across the wetted filter paper Streaked area on wetted filter paper was observed for color change to deep blue [20].
Urease test
The surface of the urea slant agar was streaked with a portion of well isolated colonies. The cap of the slant was left on loose and incubates at 35°Cfor 18-24 hours [20].
Citrate utilization test
The surface of the Simmons citrate agar slant was streaked with a portion of a well isolated colony. The cap of the slant was left on loosely and was incubates at 35°C for 18-24 hours [21].
Carbohydrate utilization test
0.1ml of a heavy saline suspension of the test organism was added to each of the four tubes containing glucose, lactose, maltose and sucrose carbohydrate disk and no to the fifth tube and was incubated at 37°C for 5 hours. It was examined at 30 minute intervals for up to 5 hours from red to yellow indicating carbohydrate utilization [22].
Analytical profile index (api) 20e test
5ml ample of API Nacl 0.85% medium was opened. Using a pipette a single well- isolated colony from an isolation plate was removed. It was carefully emulsified in 5ml ample of API Nacl 0.85% to obtained homogeneous bacterial suspension. Using the same pipette, both tube and cupule of the test CIT, VP and GEL was full with the bacterial suspension. Anaerobiosis was created in the tests ADH, LDC, ODC, H2S, and URE by overlaying with mineral oil. The incubation box was closed and incubated at 36°C for 24 hours [20].
Antimicrobial susceptibility test
Antimicrobial susceptibility test was performed on significant cultures using standard disc diffusion technique using modified Kirby-Bauer method [23]. Using a sterile loop, well isolated colonies of similar appearance was emulsified in 3-4ml of sterile physiological saline. In a good light, the turbidity of the standard was match with the turbidity of the standard, which was viewed against a printed card. Using a sterile swab, a plate of Mueller Hinton agar were inoculated (excess fluid was removed by pressing and rotating against the side of the tube). The swab was streak over the surface of the M-H agar in three directions. The surfaces of the plates were allowed to dry by allowing staying for 3-4 minute. An appropriate antimicrobial disk was evenly distributed on the inoculated plates. Within 30 minute of applying the disks. The plates were inverted and incubated appropriately depending on the organism. After overnight incubation, the control and test plates were examined to ensure the growth is confluent or near confluent. Using a ruler a zone of inhibition was measured in mm [23]. Using interpretative table, the zone sizes of each antimicrobial was measured, reporting the organism as either Sensitive (S) or Resistance(R)[23].
Results
Isolation of microorganisms
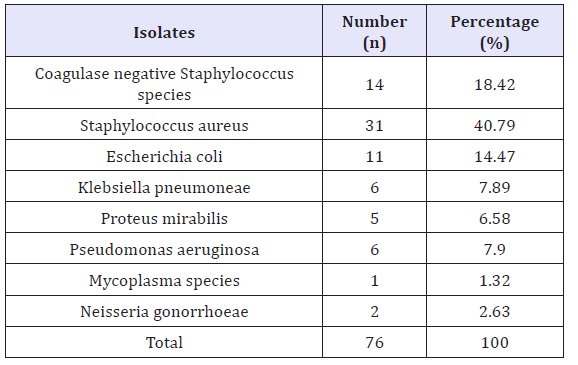
The microbial isolates obtained from seminal fluids analyzed in laboratory are presented in Table 1. The result shows a total of seventy-six (76) isolates were obtained, Staphylococcus aureuswas found to have the highest occurrence of 31 (40.79%), whereas the least was found to be Mycoplasma species, 1(1.32%). Other microorganisms encountered include; Coagulase negative Staphylococcus species14(18.42%), Escherichia coli11(14.47%),Klebsiellapneumoniae6(7.89%),Proteus mirabilis5(6.58%), Pseudomonasaeruginosa 3(3.95%) and Neisseria gonorrhoeae2(2.63%).
Table 1: Various organisms isolated and their frequencies of occurrence.
Antimicrobial susceptibility test
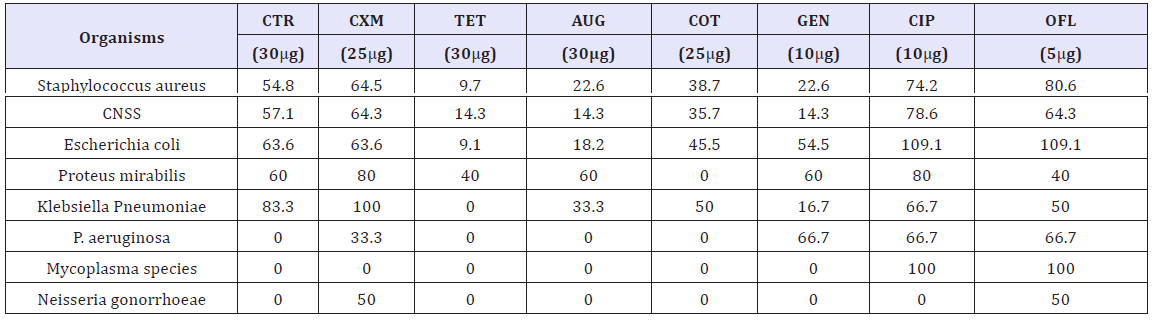
The susceptibility patterns of the bacterial isolates to various antibiotics are presented in Table 2. From the results, the isolates were most sensitive to ciprofloxacin and ofloxacin. Ceftrizone and cefuroxime however showed superior activity against most of the isolates. The activity of the Tetracycline and Augumentin against almost all the isolates was not appreciable. Cotromethazole and Gentamicin showed moderate activity against most of the isolates and no activity was observed on Mycoplasma and Neisseria gonorrheaeagainst most of the antibiotics.
Table 2: Susceptibility (%) patterns of bacterial isolates to various antibiotics Key: CNSS = Coagulase negative Staphylococcus species Key: CNSS = Coagulase negative Staphylococcus species
Susceptibility status of bacterial isolates
Table 3: Susceptibility status of bacterial isolates to various antibiotics
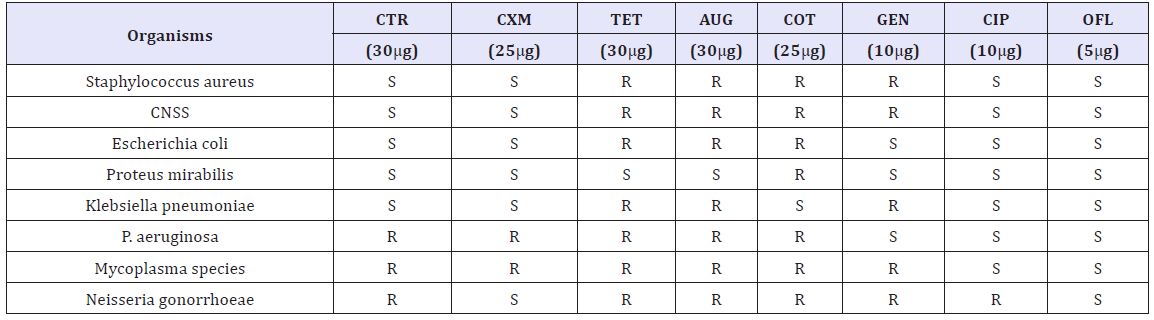
The susceptibility status of the bacterial isolates against various antibiotics used is presented in Table 3. Isolates are categorized as Sensitive (S) or Resistant (R) to the antibiotics.
Discussion
The study was aimed tocharacterized and determinethe antibiotic sensitivity pattern of bacteria isolated from semen of male patients with infertility attending Murtala Muhammad specialist hospital Kano, Nigeria From the results, a total of 76 isolates were obtained and categorized into 8 different isolates. Staphylococcus aureus was found to have the highest occurrence, 31 (40.79%), whereas the least isolate was found to be Mycoplasma species, 1(1.32%). Other microorganisms encountered include; coagulase negative Staphylococcus, 14 (18.42%), Escherichia coli11(14.47%), Klebsiella pnuemoniae6(7.89%), Proteus mirabilis 5(6.58%),Psuedomonas aeruginosa3(3.95%),Neisseria gonorrheae2(2.63%). The higher prevalence of S.Aureusin the study agrees with previous findings [6,7,9,24,25]. Komolafe and Awoniyi[25] reasoned that, the isolation of S. aureus might be associated with body hygiene of the couples involved.
Moreover, Charanchi et al. [26] contends that, the higher occurrence of S. Aureus in semen of patients with infertility could be attributed to its minimal growth requirements, high resistance to environmental factors and ability to colonize and establish infection. Conversely, the least occurrence of Mycoplasma species in this study disagrees with the work of Domes et al. [24]. Coagulase-negative Staphylococcus species are known opportunistic pathogens which are usually involved in Nosocomial and human urinary infections. Since urinary tract always act as locus of infections for the seminal tract, the heterogeneity of microorganisms encountered are capable of causing classical infections of the urogenital tract [27] and according to Ibadin and Ibeh [9] male urogenital tract infections are one of the most important causes of male infertility worldwide.
The susceptibility patterns of the isolates to various antibiotics showed that the isolates were most sensitive to ciprofloxacin and ofloxacin. Ceftrizone and Cefuroxime however showed superior activity against most of the isolates. The activity of the Tetracycline and Augumentin against the isolates was found be very poor. Cotrimethazole and Gentamicin showed moderate activity against most of the isolates and no activity was observed on Mycoplasma and Neisseria gonorrhoae against most of the antibiotics. This agrees with the works of Onemu et al. [6] as well as Komolafe and Awoniyi [25] who reported similar findings in their individual researches. The activity of Ciprofloxacin and Ofloxacin against these isolates may be due to the potent inhibition of beta-lactamase produced by most isolates especially S. aureus[28]. Similarly, the two antibiotics are Fluoroquinolones known for their high pKa and lipid solubility [8]. Moreover, poor activity of tetracycline and Augumentin may be partly influenced by the widespread misuse of these agents in Kano state as reported in a similar study by Onemu et al. [6]. Resistance to most of these antibiotics by the bacterial isolates could be due to genetic mutations and poor uptake of the antibiotics [25].
Conclusion
From the finding of this study 8 different bacterial isolates were recovered from bacteriological analysis of semen of male patients with infertility attending Murtala Muhammad Specialist Hospital (MMSH) Kano. The bacterial isolates include; Staphylococcus aureus,Mycoplasmaspecies, Coagulase negative Staphylococcus species, Escherichia coli,Klebsiella pneumoniae, Proteus mirabilis, Pseudomonasaeruginosa andNeisseria gonorrhoeae. On sensitivity testing, the isolates were most sensitive to ciprofloxacin and Ofloxacin. The activity of the Tetracycline and Augumentin against almost all the isolates was not appreciable and no activity was observed on Mycoplasma and Neisseria gonorrhoeae against most of the antibiotics.
References
- Andhoga J, Macharia AG, Maikuma IR, et al. (2002) Aerobic pathogenic bacteria in post-operative wounds at Moi Teaching and Referral Hospital. East Afr Med J 79(12): 640-644.
- Patrick JR, Frank HC, Timothy BH, Heather JM (WHO) (2010) Manual for the standardized investigation and diagnosis of the infertile couple. Cambridge University Press, Cambridge, USA, p. 92.
- Deka PK, Sarma S (2010) Psychological aspects of infertility. British Journal of Medical Practitioners 3(3): a336.
- Weng SI, Chiu MF (2014) Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS ONE 9:10.
- Diemer T, Huwe P, Ludwig M, Hauck EW, Weidner W (2003) “Urogenital infection and sperm motility. Andrologia 35(5): 283-287.
- Onemu SO, Ibeh IN (2001) Studies on the significance of positive bacterial semen cultures in male fertility in nigeria. Int J Fertil Womens Me 46(4): 210-214.
- Ekhaise, FO, Richard FR (2011) Common bacterial isolates associated with semen of men attending the fertility clinic of the University of Teaching Hospital (U.B.T.H), Benin City, Nigeria. Afr J microbial 5(22): 3805-3809.
- Keck C, Gerber SC, Clad A, Wilhelm C, Breckwoldt M (1998) Seminal tract infections: impact on male fertility and treatment options. Hum Reprod Update 4(6): 891-903.
- Ibadin O, Kai IN (2008) Bacteriospermia and sperm quality in infertile male patient at University of Benin Teaching Hospital. Benin City. Nigeria Malaysian Journal of Microbiology 4(2): 65-67.
- Askienazy E (2005) Male genital tract infection: the point of view of the bacteriologist. Gynecol Obstet Fertil 33(9): 691-697.
- Sanocka MD, Ciupinska M, Kurpis ZM (2005) Bacterial infection and semen quality. J Reprod Immunol 67(1-2): 51-56.
- Okonofua FE (2003) New reproductive technologies and infertility treatment in africa. Afr J Reprod Health 7(1): 7-8.
- Moustafa MH, Sharma RK, Thornton KJ (2004) Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Human Reproduction 19(1): 129-138.
- Al Marzoqi AH, Aboud MM, Sabri MA (2012) Study of bacterial infection associated with male infertility in Hillah city-Iraq. Journal of Biology, Agriculture and Healthcare 2(9): 10-17.
- Rowe P (2000) WHO Manual for the Standardized Investigation, Diagnosis and Management of the Infertile Male (1stedn), Cambridge: Cambridge, USA.
- Wong WY, Thomas CM, Merkus JM, Zielhius GA, Steegers RP (2000) Male factor subfertility: possible causes and impact of nutritional factors. Fertil Steril 73(3): 435-442.
- Momoh AR, Odike MA, Samuel SO, Momoh AA, Okolo PO (2007) Infertility in male: risk factors, causes and management-A review. Benin Journal of Post Graduate Medicine 9(1): 22-27.
- (2006) National Population Commission (NPC). National population census result, Abuja Nigeria.
- Enwaru CA, Iwalokam VN, Ezechi O, Oluwadun A (2016) The effect of presence of facultative bacteria species on semen and sperm quality of men seeking fertility care. African journal of urology. 22(3): 213-222.
- Chessbrough M (2006) District laboratory practice in tropical countries, (2nd edn) part two, examination of pus, ulcer material and skin specimens. Cambridge university press, pp. 80-85.
- Ochsendorf FR (2008) Sexually transmitted infections: impact on male fertility. Andrologia 40(2): 72-75.
- Holt JG, Krieg NR, Senath PHA, Staley JT, Williams ST (1994) Bergey’s Manual of Determinative Bacteriology In: Holt JG, Krieg NR, Senath PHA, Staley JT, Williams ST (Eds.), (9th edn), Baltimore Md Williams and Wilkins, USA.
- Donne C, Hershed H, Mary W, Carrofl B (2013) Microbiology Laboratory Manual Bio/2421L.
- Domes T, Lo KC, Grober ED, Mullen JB, Mazzulli T (2012) The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil Steril 97(5): 1050-1055.
- Kampole OI, Awoniyi AO (2003) Prevalence of microbial isolates associated with infertility in men attending clinics of OAUTHC, ile-ife. International journal of microbiology research and reviews. 1(5): 88-91.
- Charanchi S, Kudi A, Tahir F (2012) Antimicrobial sensitivity pattern of urogenital bacteria isolates among HIV-positive patients in the Federal Medical Center in Gombe. The Internet J Infect Dis 10(1).
- Sheikh AF and Mehdinejad M (2012) Identification and determination of coagulase negative staphylococcus specie and antimicrobial pattern of isolates from clinical specimens. Afr. J Microbial Res 16(8): 1669-1674
- Kamischke N, Nieschlag A (1999) Analysis of medical treatment of male infertility. Hum Reprod 14(1): 11-23.
© 2018 Muhammad Ali,. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)





























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






