- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Comparative Performance Evaluation of Different Brands of Ketorolac Tromethamine (NSAID’S) Generic Tablets
Sanjida Jannath, Israt Nayeema, Nishat Jahan and Kanij Nahar Deepa*
Department of Pharmacy, University of Asia Pacific, Bangladesh
*Corresponding author: Kanij Nahar Deepa, Assistant Professor, Department of Pharmacy, University of Asia Pacific, 74/A Green Road, Farmgate, Dhaka 1215, Bangladesh
Submission: March 20, 2018; Published: May 21, 2018

ISSN 2640-9275 Volume1 Issue2
Abstract
Ketorolac tromethamine is a highly potent non steroidal anti-inflammatory agent that inhibits the enzyme cyclo-oxygenase and decreases the synthesis of prostaglandins. It is mainly used for the treatment of inflammation and pain. The study was carried to compute the comparative in-vitro quality control parameters through the assessment of weight variation, friability, hardness and disintegration time and dissolution profile among the different brands of Ketorolac that are available in local market of Bangladesh. To evaluate the quality parameters, six different brands of Ketorolac 10mg tablets were selected and general quality parameters like weight variation, hardness, friability as well as in-vitro dissolution test, potency, disintegration time was carried out. Friability was found less than 1% in all brands. No noteworthy dissimilarity was found in disintegration time as they disintegrated within 5 to 30 minutes. Dissolution profile of all brands was not less than 75% of drug in 45 minutes which denotes better dissolution time in dissolution media (distilled water). It was monitored that four different brand of Ketorolac tablets were in accordance with USP guideline, except KTR3 (219.66N) and KTR6 (192.09N). The assay determines that potency of all brands was within 91.87% to 100.2%. All brands are within the limit of potency except brand KTR2 (91.87) and KTR4 (94.85) as stated by the USP specification. This study recommended that most commercially convenient Ketorolac deduced standard quality with USP specification. It also concluded that quality parameters should be maintained not only for Ketorolac but also for all kinds of medicine for getting higher quality drug products.
Introduction
Quality control is a process that ensures whether the quality of a product or service is in desired level or not. It might include necessary activities for the control and verification of definite characteristics of a product or service [1]. In a pharmaceutical sense, quality means checking and directing the degree or grade of excellence of processes and products. The purpose of this study is to ensure the product of superior efficacy, safety, and elegance and also provide physician, pharmacist and the consumer a uniform and a satisfactory product [2]. The labeled amount of drug in a formulation and its availability to the body are two important factors on which efficacy of a tablet formulation depends. Most frequent form, oral tablet is designed to deliver the drug to the human body at a specific and defined amount through the gastro-intestinal system for producing therapeutic effect. It is necessary to determine the parameters of tablets during manufacturing to ensure that the product is of desired quality [3]. For example, weight variation, hardness, friability, disintegration time, dissolution profile etc. This also includes the procedures used in the manufacturing process additionally the physiochemical properties of the active ingredients and excipients. All the mentioned parameters are firmly relevant to each other and have effect on drug bioavailability, absorption etc [4].
Ketorolac, in common with other non-steroidal antiinflammatory drugs, is an inhibitor of prostaglandin synthesis. It has antipyretic, anti-inflammatory, and analgesic activity. However, it possesses greater systemic analgesic than anti-inflammatory activity. It is administered as the tromethamine salt orally, intramuscularly, intravenously, and as a topical ophthalmic solution [5]. The chemical name for ketorolac is 5-Benzoyl-2, 3-Dihydro- 1H-Pyrolizine-1-Carboxylic Acid, 2Amino-2-(hydroxymethyl)-1, 3-Propanediol. Its sterile solution is clear and slightly yellow in color, rapidly water soluble with a molecular weight of 376.40g/ mol and its structural formula is shown in Figure 1.
Figure 1: Chemical structure of ketorolac.
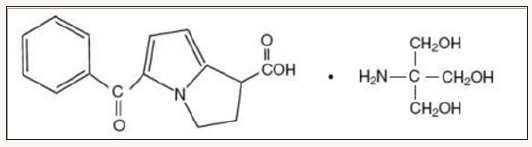
It is commonly prescribed for short-term management of moderate to moderately severe acute pain, including postsurgical pain (such as general, orthopedic and dental surgery), acute musculoskeletal trauma pain and post-partum uterine cramping pain. It may also be used for renal colic, migraine and pain due to bony metastasis [6]. Ketorolac is frequently used as analgesic drug in pregnancy and postpartum period [7]. The total duration of combined intramuscular and oral treatment should not exceed 5 days [6]. Purpose of the present study was to assess the comparative quality parameters between the tablets of nine brands having the strength of 10mg manufactured and available in Bangladesh, because better quality of medicines greatly influenced by the quality parameters.
Method and Material
Collection of sample
Wudil Local Government Area is geographically located in the south-eastern part of Kano state along Maiduguri – Kano way with a distance of about 41 Kilometer from the State capital. It is located at Latitude 110 49’ 0” N and Longitude 80 51’ 0” E. It covers an area of about 362 Km2 of land and population of about 185,189 according to 2006 census [9]. Wudil Local Government shares common boundaries with Warawa (North), Dawakin-kudu (West), Garko (South) and Gaya Local Government (East).
Sample collection
Table 1: Label information of six different brands of ketorolac tablets.
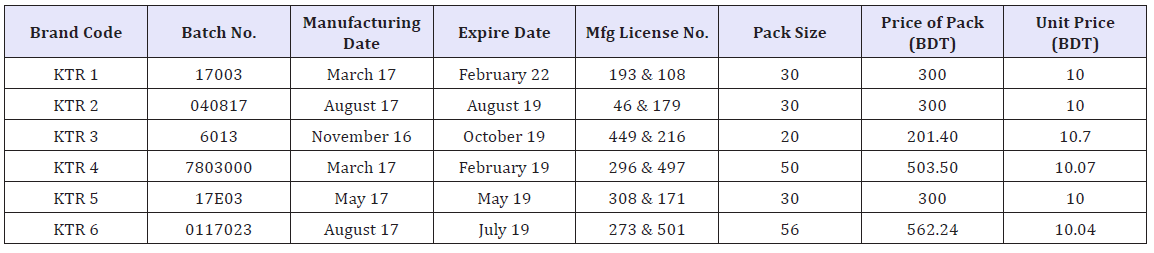
There are approximately 47 different brands of Ketorolac available in local market in Bangladesh (according to BD.DRUGS. COM). Among them the marketed sample of 6 (Six) brands (about 20 tablets of each brand) of Ketorolac or Ketorolac Tromethanine (10mg) tablet were purchased at M.R.P from Retail pharmacy at Dhaka in Bangladesh. The samples were randomly coded as KTR1, KTR2, KTR3, KTR4, KTR5, KTR6 so that the identity of the manufacture can be blinded. The samples were properly checked at the time of purchase for their physical appearance, name of manufacturer, manufacturing date, manufacturing license number, batch number, expiry date, DAR number & maximum retail price (Table 1). At the beginning of the research work, visual inspection is done for the color, size and shape of the tablets.
Chemical reagents
No other reagent without distilled water was used. And this distilled water (media) was collected from the Project Laboratory of Pharmacy department of University of Asia Pacific.
Weight variation test
Non-uniform weight may result in dose variation. Variation of weight from tablet to tablet, capsule to capsule can be determined by this process. Arbitrarily 20 tablets were picked for individual brand like X1, X2, X3 ….. Xn and weighted using an analytical balance named Pioneer, OHAUS, USA. The following formula was used to determine the average weight [8]: Average weight= Total weight of all tablet/ No. of tablets
Then the ± % deviations were determined by using the following formulas:
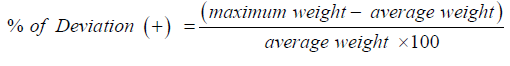
Hardness test
The hardness of the tablet depends on the materials used, amount of binder, the space between the upper and lower punches at the time of compression and pressure applied during the process of compression [9]. This test was performed for at least three Ketorolac tablets of each brand. Hardness generally measures the tablet crushing strength. Hardness affects the disintegration process. Too hard tablets may not disintegrate in the required period of time and similarly, too soft tablets may not withstand the handling during subsequent processing such as coating or packaging [10].
The average hardness is calculated by following way:
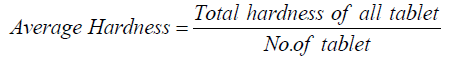
Friability test
Problems like chipping, capping, lamination can be produced when compressed into very hard tablets. So, tablet hardness is not an absolute indicator of strength. To determine the ability of tablets to withstand abrasion during packaging, handling, and shipping processes, friability test is performed. A maximum weight loss of not more than 1% is generally considered acceptable [11]. From each brand 7 tablets were weighed and tumbled into friabilator at 25rpm for 4minutes (100 revolutions). Then Ketorolac tablets were reweighted. The loss of weight is determined by following way:
Loss of weight = (Initial weight − Final weight )This loss of weight indicates the friability of Ketorolac tablet. Finally the percent of weight of loss was calculated by following way [12]:
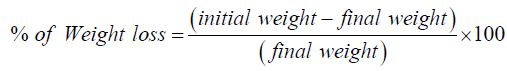
Disintegration test
The rate of absorption of a drug is affected by the dissolution rate of a drug which depends on the disintegration time of that drug. The API solubility in the gastric fluids of the digestive system can be easily known by disintegration test. This test is ideal for all tablets but is not performed for controlled and sustained release tablets [13]. Disintegration Tester was used and the chief instrument for the test with disc in distilled water medium. In each tube 3 tablets of each brand were placed and the basket rack is positioned in a 1000ml beaker of water at 37±0.5 °C. When no particle remained on the basket of the system, that disintegration time was taken as the time. Then the mean disintegration time was calculated for every brand.
Potency test
Potency test is done in tablets is to evaluate the tablet’s potential for efficacy. 10 tablets of each brand were taken and their average weight was determined, then they were crushed and the amount of powdered tablet equivalent to 10mg of Ketorolac drug was determined by calculation. Determined amount of powder was taken in a volumetric flask (100ml) and distilled water was added up to 100ml. This solution was filtered and from this, 10ml filtrate was taken in a test tube and again 100ml of distilled water was added to dilute it. Absorbance of the sample was determined at 317nm wavelength by using UV-visible spectrophotometer. Finally the potency of different tablets was calculated by using the following equation [14]:

Dissolution test
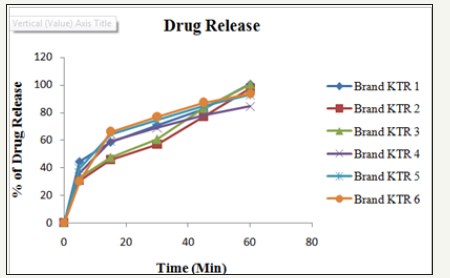
The dissolution test was undertaken for six randomly selected tablets using dissolution USP II dissolution apparatus (paddle) at 50rmp and 600ml of distilled water at 37 °C. Samples were withdrawn at different times (5, 15, 30, 45, 60minutes), filtered with quantitative filter paper and diluted with distilled water (dissolution media). Absorbance was measured at 317nm by UVVIS spectrophotometer (UV-1700, Shimadzu, Japan). Finally the percent release of Ketorolac tablet was determined by following equation [14].
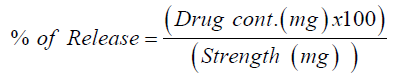
Result and Discussion
Weight variation
Uniformity of weight is important in terms of good manufacturing practice. Factors such as head pressure, tooling of the compression machine, flow properties of the powder and machine speed etc. can affect the uniformity of tablets [15]. It is desirable that all the dosage forms such as tablets; capsules etc of a particular batch should be uniform in weight because non-uniform weight may lead to dose variation. According to USP specification for uniformity of weight which states that tablets weighing 130- 324mg, not more than 2 tablets should not differ from the average weight by more than ±7.5% [12]. All brands assure their uniformity of weight with the specification according to USP (Table 2).
Table 2: Potency and weight variation of six different brands of ketorolac tablet collected from local market in bangladesh.
Hardness test
The hardness of six brands of Ketorolac tablets was measured by using an automatic tablet hardness tester (Dr. Schleuniger, Model 8X, Switzerland) and the observed results are compiled in the (Table 3).
Table 3: Hardness, friability and disintegration of six different brands of ketorolac tablet.
Friability test
The tendency of a solid substance to break into smaller pieces under pressure is considered as friability. The strength of the tablets to endure coating, packaging, transport and shipping is evaluated by this test. The Pharmacopoeia specification for friability is not more than 1% [12]. The result of the tablet friability test showed that all the brands tested had percent friability below 1% (Table 3).
Disintegration test
Disintegration is the first step towards dissolution process which shows the breakdown of tablet into smaller particles. To find out the time it takes for a solid oral dosage form like a tablet or capsule to completely disintegrate, the disintegration test is performed. The time of disintegration is a measure of the quality. According to USP specification tablets should disintegrate in 5 to 30minutes [12]. The test was conducted on the six different brands of Ketorolac tablets. All the tablets that have been tested are within the limit (Table3).
<Potency test
The assay determines that potency of all brands was within 91.9% to 100.2%. USP specification for the drugs is not less than 95.0% and not more than 105.0% [12]. All brands are within the limit of potency except brand KTR2 (91.9) and KTR4 (94.85) according to the USP specification (Table 2).
Table 4: Average drug release (%) of six different brands of ketorolac tablets.
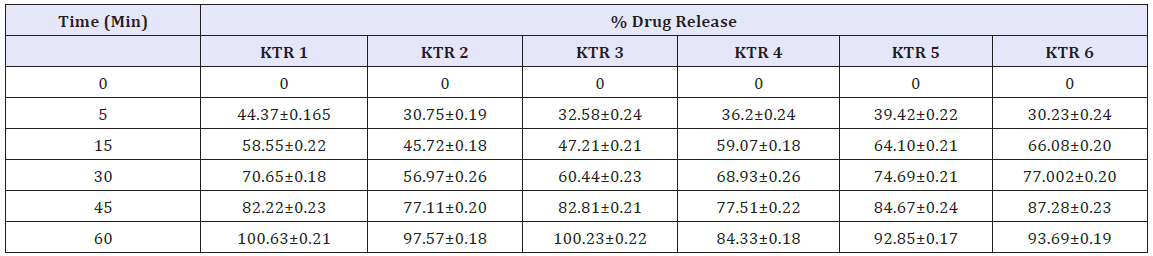
Dissolution test
To determine the dissolution properties of the active drug and the percent release of the samples in a specific time, dissolution test is a must [16]. The dissolution study of Ketorolac tablet was carried out in water media. In this study, dissolution profiles of six brands were determined to provide information regarding biological bioavailability and batch to batch consistency (Figures 2-6). The result complies with the requirement of USP which states not less than 75% of the labeled amount dissolved in 45minutes [12] (Table 4).
Figure 2: Potency of six different brands of ketorolac tablet.
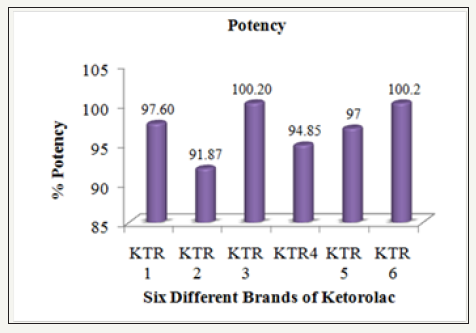
Figure 3: Average hardness of six different brands of ketorolac tablet
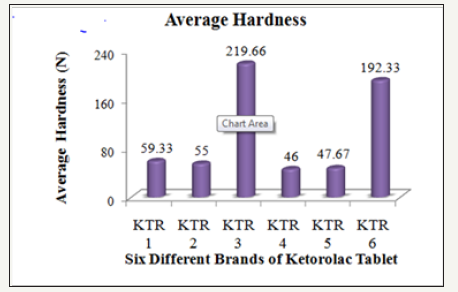
Figure 4:Friability of six different brands of ketorolac tablet.
Figure 5: Average disintegration time of six different brands of ketorolac tablet
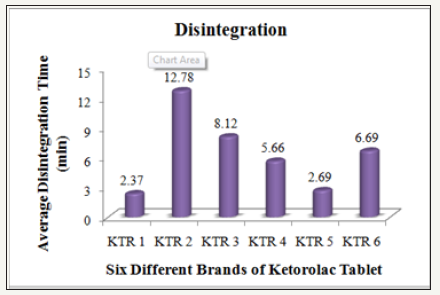
Figure 6: Drug release (%) of six different brands of ketorolac.
Conclusion
Ketorolac is a widely used drug in Bangladesh. The study was undertaken with an aim to evaluate different brands of Ketorolac market preparations available in Bangladesh. We choose six different brands from different pharmaceutical company in Bangladesh. We performed various official and non-official and in-vitro studies like weight variation, thickness measurement, friability, hardness, dis integration, dissolution and potency tests. According to our analysis, almost all brands of Ketorolac contain good quality and good efficacy by passing all the pharmacopoeia requirements. But only a few tablets showed slightly dissimilar result as compared to U.S.P. specification. Bioavailability and therapeutic effect depends on these quality control tests. These types of studies should be conducted more frequently not only to build public awareness about the quality of marketed pharmaceutical products but also because these type of studies are very helpful for the advancement of our pharmaceutical sector. Sub-standard drugs manufacturing can also be prevented by conducting this study.
References
- Hasan A, Dewan SAR, Ahamed SK, Kai MM (2013) Quality control studies on cetirizine hydrochloride tablets available in Bangladesh drug market. International Journal of Pharmacy and Biological Sciences 3(1): 349- 354.
- Nasrin N, Asaduzzaman M, Mowla R, Rizwan F, Alam A (2011) A comparative study of physical parameters of selected ketorolac tromethamine tablets available in the pharma market of Bangladesh. Journal of Applied Pharmaceutical Science 1(8): 101-103.
- Afifi SA, Ahmadeen S (2012) A comparative study for evaluation of different brands of metformin hydrochloride 500mg tablets marketed in Saudi Arabia. Life Science Journal 9(4): 4260-4266.
- Karmakar P, Kibria G (2012) In-vitro comparative evaluation of quality control parameters between paracetamol and paracetamol/caffeine tablets available in Bangladesh. International Current Pharmaceutical Journal 1(5): 103-109.
- Buckley MMT, Brogden RN (1990) Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs 39(1): 86-109.
- Tripathi KD (2013) Autacoids and related drugs, essential of medical pharmacology. Jaypee Brothers Medical Publishers, New Delhi, India, pp. 200-202.
- Soldin OP, Mattison DR (2009) Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 48(3): 143-157.
- Nushrat SA, Nasreen W, Deepa KN (2017) In vitro comparative study of quality control parameters of some brand of Ketorolac tablets available in Bangladesh. World Journal of Pharmacy and Pharmaceutical Sciences 6(8): 146-155.
- Banker GS, Anderson NR, In Lachman L, Lieberman HA, Kanig JL (2011) The theory and practice of industrial pharmacy. Varghese Publishing house, USA, pp. 293-345.
- Shabana MD (2016) Review on the quality control analysis of oral dosage form: tablets. Journal of Pharmacy and Pharmaceutical Sciences 5(2): 108-114.
- Kumar D, Singh J, Antil M, Kumar V (2016) Quality control of tablets: A review. International Journal of Universal Pharmacy and Bio Sciences 5(4): 53-67.
- Lachman L, Lieberman HA (2013) Pharmaceutical dosage forms. In: Lachman L, Lieberman HA, Khar RK, Vyas SP, Ahmed FJ, Jain GK (Eds.), CBS Publishers & Distributors, India, pp. 449-561.
- Hirani AJ, Rathod D, Vadalia KR (2009) Orally disintegrating tablets: a review. Tropical Journal of Pharmaceutical Research 8(2): 161-172.
- Das P, Das S, Akhter R, Huque S, Akter R, et al. (2017) Comparative in vitro equivalence evaluation of some spironolactone generic tablets, commercially available in Bangladesh drug market. Indo American Journal of Pharmaceutical Research 7(10):
- Narasimharao R, Somashekar S, Prakash K (2011) Design and evaluation of Keterolac tromethamine impregnated hydroxy propyl methyl cellulose films. Journal of Chemical and Pharmaceutical Research 3(1): 360- 381.
- (2010) The United States Pharmacopeia (USP) The United States pharmacopeial convention: 12601 Twinbrook Parkway, Rockville, USA 29(6): 19-15.
© 2018 Kanij Nahar Deepa. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






